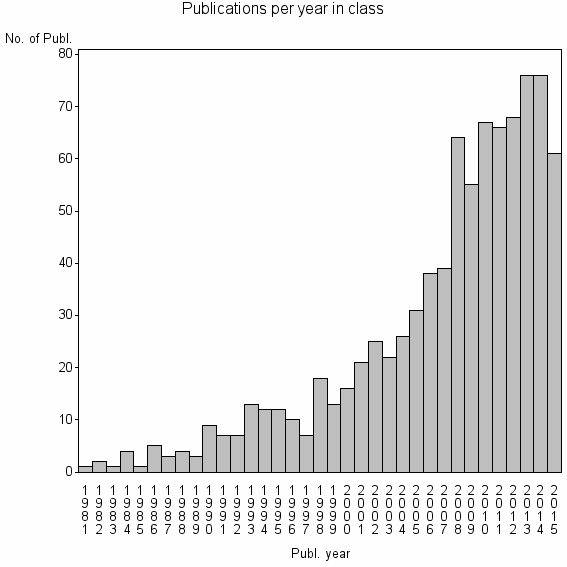
Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
| 11877 | 883 | 26.9 | 72% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 970 | 10197 | CAUSAL INFERENCE//PRINCIPAL STRATIFICATION//STATISTICS IN MEDICINE |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | CONTINUAL REASSESSMENT METHOD | Author keyword | 188 | 89% | 10% | 84 |
| 2 | DOSE FINDING | Author keyword | 68 | 47% | 12% | 107 |
| 3 | DOSE FINDING STUDIES | Author keyword | 33 | 74% | 3% | 25 |
| 4 | PHASE I TRIALS | Author keyword | 28 | 43% | 6% | 49 |
| 5 | MAXIMUM TOLERATED DOSE | Author keyword | 20 | 29% | 7% | 59 |
| 6 | ESCALATION WITH OVERDOSE CONTROL | Author keyword | 17 | 100% | 1% | 8 |
| 7 | UP AND DOWN DESIGN | Author keyword | 15 | 88% | 1% | 7 |
| 8 | BAYESIAN ADAPTIVE DESIGN | Author keyword | 12 | 63% | 1% | 12 |
| 9 | PHASE I TRIAL | Author keyword | 11 | 18% | 7% | 58 |
| 10 | PHASE 1 TRIALS | Author keyword | 10 | 61% | 1% | 11 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | CONTINUAL REASSESSMENT METHOD | 188 | 89% | 10% | 84 | Search CONTINUAL+REASSESSMENT+METHOD | Search CONTINUAL+REASSESSMENT+METHOD |
| 2 | DOSE FINDING | 68 | 47% | 12% | 107 | Search DOSE+FINDING | Search DOSE+FINDING |
| 3 | DOSE FINDING STUDIES | 33 | 74% | 3% | 25 | Search DOSE+FINDING+STUDIES | Search DOSE+FINDING+STUDIES |
| 4 | PHASE I TRIALS | 28 | 43% | 6% | 49 | Search PHASE+I+TRIALS | Search PHASE+I+TRIALS |
| 5 | MAXIMUM TOLERATED DOSE | 20 | 29% | 7% | 59 | Search MAXIMUM+TOLERATED+DOSE | Search MAXIMUM+TOLERATED+DOSE |
| 6 | ESCALATION WITH OVERDOSE CONTROL | 17 | 100% | 1% | 8 | Search ESCALATION+WITH+OVERDOSE+CONTROL | Search ESCALATION+WITH+OVERDOSE+CONTROL |
| 7 | UP AND DOWN DESIGN | 15 | 88% | 1% | 7 | Search UP+AND+DOWN+DESIGN | Search UP+AND+DOWN+DESIGN |
| 8 | BAYESIAN ADAPTIVE DESIGN | 12 | 63% | 1% | 12 | Search BAYESIAN+ADAPTIVE+DESIGN | Search BAYESIAN+ADAPTIVE+DESIGN |
| 9 | PHASE I TRIAL | 11 | 18% | 7% | 58 | Search PHASE+I+TRIAL | Search PHASE+I+TRIAL |
| 10 | PHASE 1 TRIALS | 10 | 61% | 1% | 11 | Search PHASE+1+TRIALS | Search PHASE+1+TRIALS |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | CONTINUAL REASSESSMENT METHOD | 204 | 60% | 25% | 220 |
| 2 | I CLINICAL TRIALS | 70 | 52% | 11% | 97 |
| 3 | I II CLINICAL TRIALS | 49 | 94% | 2% | 17 |
| 4 | I TRIALS | 44 | 85% | 3% | 23 |
| 5 | LATE ONSET TOXICITIES | 38 | 76% | 3% | 26 |
| 6 | MOLECULARLY TARGETED AGENTS | 27 | 92% | 1% | 11 |
| 7 | PHASE I TRIALS | 22 | 43% | 5% | 40 |
| 8 | ONCOLOGY TRIALS | 21 | 48% | 4% | 31 |
| 9 | OVERDOSE CONTROL | 17 | 72% | 1% | 13 |
| 10 | DOWN DESIGN | 14 | 64% | 2% | 14 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| Designs of drug-combination phase I trials in oncology: a systematic review of the literature | 2015 | 1 | 28 | 75% |
| Dose Escalation Methods in Phase I Cancer Clinical Trials | 2009 | 109 | 73 | 59% |
| Adaptive designs for dual-agent phase I dose-escalation studies | 2013 | 4 | 72 | 85% |
| Life-expectancy of patients enrolled in phase 1 clinical trials: A systematic review of published prognostic models | 2012 | 5 | 20 | 95% |
| Principles of dose finding studies in cancer: a comparison of trial designs | 2013 | 2 | 28 | 86% |
| Meta-analysis of the Relationship Between Dose and Benefit in Phase I Targeted Agent Trials | 2012 | 8 | 12 | 50% |
| Experimental designs for phase I and phase I/II dose-finding studies | 2006 | 31 | 13 | 69% |
| A New Model for Determining the MTD During Phase-I Trials in Pediatric Oncology | 2012 | 3 | 8 | 75% |
| ICH S9: Developing Anticancer Drugs, One Year Later | 2011 | 3 | 5 | 80% |
| Phase I studies of chemotherapeutic agents in cancer patients: A review of the designs | 2006 | 15 | 65 | 89% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | TRANSLAT PL STAT | 6 | 53% | 0.9% | 8 |
| 2 | UNIT EA2694 | 4 | 75% | 0.3% | 3 |
| 3 | UNIT MOL THER Y CANC | 3 | 100% | 0.3% | 3 |
| 4 | ETH HUMANITIES SPIRITUAL CARE | 3 | 30% | 0.8% | 7 |
| 5 | QUAL LIFE PALLIAT CARE | 2 | 44% | 0.5% | 4 |
| 6 | PL DEV SUPPORT DIRECTORATE | 2 | 36% | 0.6% | 5 |
| 7 | PHASE CLIN TRIALS PROGRAM | 2 | 67% | 0.2% | 2 |
| 8 | UNITE 436 | 2 | 67% | 0.2% | 2 |
| 9 | EQUIPE ACCUEIL ST PUBL EPIDEMIOL MODELISAT MALA | 2 | 50% | 0.3% | 3 |
| 10 | MED PHARMACEUT STAT UNIT | 2 | 12% | 1.6% | 14 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000103116 | CONDITIONAL POWER//INTERIM ANALYSIS//SAMPLE SIZE RE ESTIMATION |
| 2 | 0.0000097151 | PATIENT RECRUITMENT//MINORITY RECRUITMENT//TRIAL PARTICIPATION |
| 3 | 0.0000083959 | INTERACTION CONDITION//TARGETED CLINICAL TRIALS//INFLUENCE CONDITION |
| 4 | 0.0000080732 | D OPTIMALITY//APPROXIMATE DESIGNS//APPROXIMATE DESIGN |
| 5 | 0.0000060964 | RESPONSE ADAPTIVE DESIGN//DOUBLY ADAPTIVE BIASED COIN DESIGN//RESPONSE ADAPTIVE DESIGNS |
| 6 | 0.0000058009 | MANAGEMENT HEALTHCARE//RESEARCH NURSE//CLINICAL RESEARCH COORDINATORS |
| 7 | 0.0000057718 | EQUIPOISE//SHAM SURGERY//DEVELOPING WORLD BIOETHICS |
| 8 | 0.0000054420 | KLIN DERMATOL DERMATOL ALLERGOL//COLLECTIVE ETHICS//DRUG EXAMINATION |
| 9 | 0.0000051195 | SERV PSYCHIAT MENTAL HLTH//SYMPTOMATIC VOLUNTEERS//UNDUE INDUCEMENT |
| 10 | 0.0000048829 | ASSENT//RESEARCH PARTICIPATION DECISION MAKING//NON THERAPEUTIC RESEARCH |