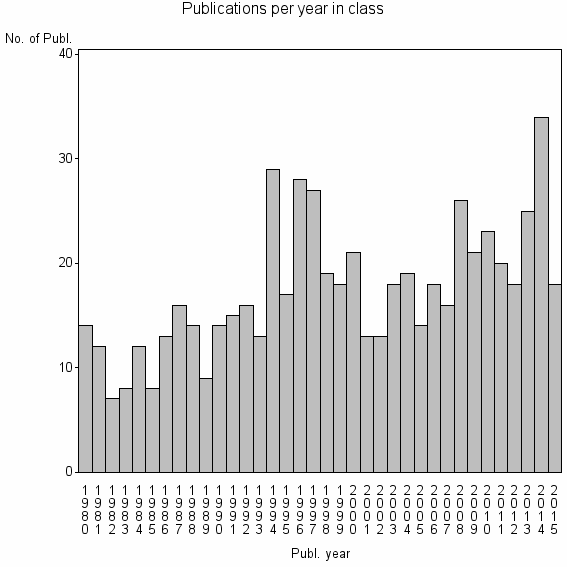
Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
| 15717 | 626 | 39.9 | 26% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 1294 | 8163 | FOOD AND DRUG LAW JOURNAL//GENERIC SUBSTITUTION//DIRECT TO CONSUMER ADVERTISING |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | FOOD AND DRUG LAW JOURNAL | Journal | 59 | 23% | 35% | 222 |
| 2 | HUMANITARIAN DEVICE EXEMPTION | Author keyword | 2 | 67% | 0% | 2 |
| 3 | PREMARKET APPROVAL | Author keyword | 2 | 36% | 1% | 4 |
| 4 | GLOBAL SUPPLY | Address | 1 | 38% | 0% | 3 |
| 5 | INVESTIGATIONAL DEVICE EXEMPTION | Author keyword | 1 | 38% | 0% | 3 |
| 6 | CYBERSURGERY | Author keyword | 1 | 100% | 0% | 2 |
| 7 | LABORATORY REGULATION | Author keyword | 1 | 50% | 0% | 2 |
| 8 | MEDICAL DEVICE AMENDMENTS | Author keyword | 1 | 100% | 0% | 2 |
| 9 | MEDICAL DEVICE INNOVATION | Author keyword | 1 | 100% | 0% | 2 |
| 10 | QA COMPLIANCE VALIDAT | Address | 1 | 100% | 0% | 2 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | HUMANITARIAN DEVICE EXEMPTION | 2 | 67% | 0% | 2 | Search HUMANITARIAN+DEVICE+EXEMPTION | Search HUMANITARIAN+DEVICE+EXEMPTION |
| 2 | PREMARKET APPROVAL | 2 | 36% | 1% | 4 | Search PREMARKET+APPROVAL | Search PREMARKET+APPROVAL |
| 3 | INVESTIGATIONAL DEVICE EXEMPTION | 1 | 38% | 0% | 3 | Search INVESTIGATIONAL+DEVICE+EXEMPTION | Search INVESTIGATIONAL+DEVICE+EXEMPTION |
| 4 | CYBERSURGERY | 1 | 100% | 0% | 2 | Search CYBERSURGERY | Search CYBERSURGERY |
| 5 | LABORATORY REGULATION | 1 | 50% | 0% | 2 | Search LABORATORY+REGULATION | Search LABORATORY+REGULATION |
| 6 | MEDICAL DEVICE AMENDMENTS | 1 | 100% | 0% | 2 | Search MEDICAL+DEVICE+AMENDMENTS | Search MEDICAL+DEVICE+AMENDMENTS |
| 7 | MEDICAL DEVICE INNOVATION | 1 | 100% | 0% | 2 | Search MEDICAL+DEVICE+INNOVATION | Search MEDICAL+DEVICE+INNOVATION |
| 8 | CDRH | 1 | 40% | 0% | 2 | Search CDRH | Search CDRH |
| 9 | DEVICE REGULATION | 1 | 40% | 0% | 2 | Search DEVICE+REGULATION | Search DEVICE+REGULATION |
| 10 | DEVICE APPROVAL | 1 | 25% | 0% | 3 | Search DEVICE+APPROVAL | Search DEVICE+APPROVAL |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | PREMARKET APPROVAL | 11 | 69% | 1% | 9 |
| 2 | FEDERAL PREEMPTION | 7 | 57% | 1% | 8 |
| 3 | CARDIOVASCULAR DEVICES | 5 | 39% | 2% | 11 |
| 4 | COSMETIC ACT | 4 | 50% | 1% | 6 |
| 5 | GOVERNMENT REGULATION | 3 | 39% | 1% | 7 |
| 6 | FDAS | 3 | 57% | 1% | 4 |
| 7 | ICD TECHNOLOGY | 2 | 67% | 0% | 2 |
| 8 | APPROVAL PROCESS | 2 | 26% | 1% | 6 |
| 9 | CART PROGRAM | 1 | 50% | 0% | 2 |
| 10 | HUMANITARIAN DEVICE EXEMPTION | 1 | 100% | 0% | 2 |
Journals |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | FOOD AND DRUG LAW JOURNAL | 59 | 23% | 35% | 222 |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| Strength of Study Evidence Examined by the FDA in Premarket Approval of Cardiovascular Devices | 2009 | 64 | 7 | 29% |
| How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review | 2012 | 12 | 11 | 82% |
| Characteristics of Pivotal Trials and FDA Review of Innovative Devices | 2015 | 1 | 6 | 50% |
| The architecture of government regulation of medical products | 1996 | 71 | 24 | 58% |
| The prescription drug user fee act: Is a faster food and drug administration always a better food and drug administration? | 2005 | 12 | 23 | 70% |
| THE FEDERAL-REGULATION OF MEDICAL DEVICES | 1987 | 41 | 21 | 90% |
| Review of the Processes for FDA Oversight of Drugs, Medical Devices, and Combination Products | 2011 | 13 | 12 | 58% |
| Losing deference in the FDA's second century: Judicial review, politics, and a diminished legacy of expertise | 2008 | 14 | 17 | 59% |
| A full-fledged overhaul is needed for a risk and value-based regulation of medical devices in Europe | 2013 | 4 | 11 | 36% |
| A critical examination of the FDA's efforts to preempt failure-to-warn claims | 2008 | 30 | 11 | 27% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | GLOBAL SUPPLY | 1 | 38% | 0.5% | 3 |
| 2 | QA COMPLIANCE VALIDAT | 1 | 100% | 0.3% | 2 |
| 3 | VA EASTERN KANSAS HLTH CARE SYST | 1 | 50% | 0.3% | 2 |
| 4 | MED DEVICE SAFETY | 1 | 40% | 0.3% | 2 |
| 5 | INTERDISCIPLINARY HLTH TECHNOL ASSESSMENT HTA | 1 | 25% | 0.5% | 3 |
| 6 | HYDRODYNAM ACOUST BRANCH | 1 | 33% | 0.3% | 2 |
| 7 | CARDIOVASC P NEUROL DEVICES | 1 | 50% | 0.2% | 1 |
| 8 | CERTS COORDINATING | 1 | 50% | 0.2% | 1 |
| 9 | HSRD VA STROKE QUERI | 1 | 50% | 0.2% | 1 |
| 10 | OFF ENFORCEMENT | 1 | 50% | 0.2% | 1 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000190470 | FOOD AND DRUG LAW JOURNAL//ADVOCACY NETWORK//AGR LAW |
| 2 | 0.0000164887 | INTELLECTUAL PROPERTY PROGRAM//CARDIORENAL DRUGS//FOOD DRUG GRP |
| 3 | 0.0000158644 | DRUG LAG//OFF PHARMACEUT IND//JAPANESE CLINICAL TRIALS |
| 4 | 0.0000141175 | DIRECT TO CONSUMER ADVERTISING//DTCA//DTC ADVERTISING |
| 5 | 0.0000084143 | LOUISIANA LAW REVIEW//TORT & INSURANCE LAW JOURNAL//PUNITIVE DAMAGES |
| 6 | 0.0000077518 | LIVE SURGERY//LIVE CASE DEMONSTRATION//BROADGREEN UNIV HOSP |
| 7 | 0.0000064838 | UNLICENSED//OFF LABEL//OFF LABEL DRUG USE |
| 8 | 0.0000061630 | BIC GRANADA//HERBAL NANOPARTICLES//RAJAH MUTHAIAH MED HOSP |
| 9 | 0.0000058644 | MULTIPLE INDUSTRIES//RETURN ON INNOVATION//VALUE TRANSFERENCE |
| 10 | 0.0000050182 | ORPHAN DRUGS//RARE DISEASES//OFF ORPHAN PROD DEV |