Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
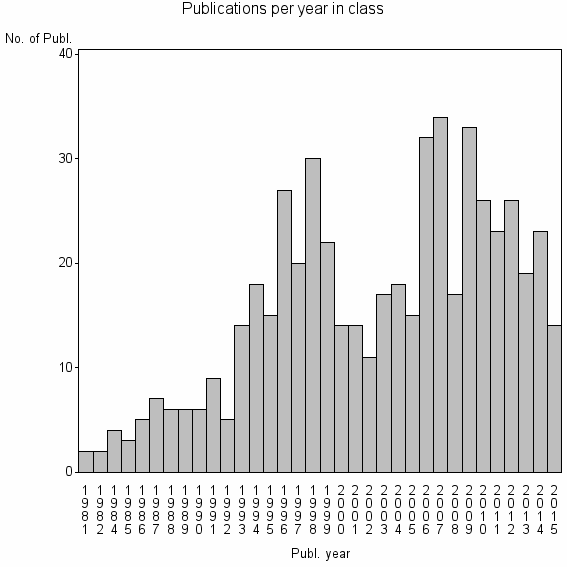
| 17413 | 537 | 21.1 | 50% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 2436 | 3630 | ACCREDITATION AND QUALITY ASSURANCE//PROFICIENCY TESTING//MEASUREMENT UNCERTAINTY |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | ACCURACY PROFILE | Author keyword | 20 | 48% | 6% | 30 |
| 2 | ANALYTICAL METHOD TRANSFER | Author keyword | 18 | 89% | 1% | 8 |
| 3 | LERMDV | Address | 11 | 100% | 1% | 6 |
| 4 | TOTAL ERROR | Author keyword | 9 | 36% | 4% | 21 |
| 5 | ROBUSTNESS TEST | Author keyword | 6 | 42% | 2% | 11 |
| 6 | BETA EXPECTATION TOLERANCE INTERVAL | Author keyword | 5 | 50% | 1% | 7 |
| 7 | BIOANALYT CHEM UNIT | Address | 3 | 50% | 1% | 5 |
| 8 | TOLERANCE INTERVAL | Author keyword | 3 | 21% | 3% | 15 |
| 9 | PHARMACEUT DEV SCI 2 | Address | 3 | 100% | 1% | 3 |
| 10 | RUGGEDNESS TEST | Author keyword | 3 | 42% | 1% | 5 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | ACCURACY PROFILE | 20 | 48% | 6% | 30 | Search ACCURACY+PROFILE | Search ACCURACY+PROFILE |
| 2 | ANALYTICAL METHOD TRANSFER | 18 | 89% | 1% | 8 | Search ANALYTICAL+METHOD+TRANSFER | Search ANALYTICAL+METHOD+TRANSFER |
| 3 | TOTAL ERROR | 9 | 36% | 4% | 21 | Search TOTAL+ERROR | Search TOTAL+ERROR |
| 4 | ROBUSTNESS TEST | 6 | 42% | 2% | 11 | Search ROBUSTNESS+TEST | Search ROBUSTNESS+TEST |
| 5 | BETA EXPECTATION TOLERANCE INTERVAL | 5 | 50% | 1% | 7 | Search BETA+EXPECTATION+TOLERANCE+INTERVAL | Search BETA+EXPECTATION+TOLERANCE+INTERVAL |
| 6 | TOLERANCE INTERVAL | 3 | 21% | 3% | 15 | Search TOLERANCE+INTERVAL | Search TOLERANCE+INTERVAL |
| 7 | RUGGEDNESS TEST | 3 | 42% | 1% | 5 | Search RUGGEDNESS+TEST | Search RUGGEDNESS+TEST |
| 8 | METHOD CAPABILITY | 2 | 67% | 0% | 2 | Search METHOD+CAPABILITY | Search METHOD+CAPABILITY |
| 9 | SYSTEM SUITABILITY TESTS | 2 | 67% | 0% | 2 | Search SYSTEM+SUITABILITY+TESTS | Search SYSTEM+SUITABILITY+TESTS |
| 10 | ROBUSTNESS TESTING | 2 | 16% | 2% | 10 | Search ROBUSTNESS+TESTING | Search ROBUSTNESS+TESTING |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | U 98 528 | 33 | 100% | 2% | 13 |
| 2 | DECISION CRITERION | 21 | 90% | 2% | 9 |
| 3 | RUGGEDNESS TESTS | 17 | 62% | 3% | 18 |
| 4 | TOTAL ERROR | 17 | 68% | 3% | 15 |
| 5 | RUGGEDNESS TEST | 12 | 75% | 2% | 9 |
| 6 | HPLC METHOD VALIDATION | 11 | 65% | 2% | 11 |
| 7 | QUANTITATIVE ANALYTICAL PROCEDURES | 10 | 26% | 6% | 32 |
| 8 | SFSTP PROPOSAL | 9 | 24% | 6% | 32 |
| 9 | ACCURACY PROFILES | 7 | 64% | 1% | 7 |
| 10 | WASHINGTON CONFERENCE | 5 | 60% | 1% | 6 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references | % act. ref. to same field |
|---|---|---|---|---|
| Advances in validation, risk and uncertainty assessment of bioanalytical methods | 2011 | 42 | 86 | 40% |
| Validation of bioanalytical chromatographic methods | 1998 | 154 | 35 | 49% |
| Analytical method transfer using equivalence tests with reasonable acceptance criteria and appropriate effort: Extension of the ISPE concept | 2010 | 7 | 4 | 100% |
| Capsule review on bioanalytical method transfer: opportunities and challenges for chromatographic methods | 2011 | 6 | 6 | 100% |
| Methodologies for the transfer of analytical methods: A review | 2009 | 10 | 34 | 71% |
| Method validation across the disciplines-Critical investigation of major validation criteria and associated experimental protocols | 2009 | 21 | 28 | 43% |
| The transfer of analytical procedures | 2013 | 1 | 10 | 70% |
| Ruggedness and robustness testing | 2007 | 104 | 109 | 24% |
| Review of statistical methodologies used to compare (bio)assays | 2009 | 7 | 14 | 57% |
| Chromatographic Procedures in a Regulated Environment | 2011 | 0 | 10 | 90% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | LERMDV | 11 | 100% | 1.1% | 6 |
| 2 | BIOANALYT CHEM UNIT | 3 | 50% | 0.9% | 5 |
| 3 | PHARMACEUT DEV SCI 2 | 3 | 100% | 0.6% | 3 |
| 4 | ANALYT CHEM CHU | 2 | 67% | 0.4% | 2 |
| 5 | INTER ULTAIRE RECH MEDICAMENT | 2 | 67% | 0.4% | 2 |
| 6 | CHEMOAC | 2 | 12% | 2.8% | 15 |
| 7 | GLOBAL ANALYT DEV | 2 | 24% | 1.1% | 6 |
| 8 | LILLY DEV | 2 | 33% | 0.7% | 4 |
| 9 | CHUCIRM | 1 | 38% | 0.6% | 3 |
| 10 | DEV SLOVENIA | 1 | 50% | 0.4% | 2 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000253941 | OPERATIONAL QUALIFICATION//PERFORMANCE QUALIFICATION//DESIGN QUALIFICATION |
| 2 | 0.0000130319 | ACCREDITATION AND QUALITY ASSURANCE//PROFICIENCY TESTING//PROFICIENCY TEST |
| 3 | 0.0000114134 | TOLNAFTATE//KETOCONAZOLE DETERMINATION//PIRIBEDIL |
| 4 | 0.0000112289 | COMPUTER ASSISTED METHOD DEVELOPMENT//GRADIENT ELUTION//CHROMATOGRAPHIC RESPONSE FUNCTION CRF |
| 5 | 0.0000106789 | PREDICTION DISTRIBUTION//MARGINAL LIKELIHOOD FUNCTION//CLIN BIOSTAT 2 |
| 6 | 0.0000104982 | BIOANALYSIS//BIOANALYT METAB PHARMACOKINET//CASSETTE ANALYSIS |
| 7 | 0.0000101890 | KNOWLEDGE DOMAIN MATRIX//CHROMATOGRAM BASE//COASTAL MEASUREMENTS |
| 8 | 0.0000095142 | H POINT STANDARD ADDITION METHOD//HPSAM//H POINT STANDARD ADDITIONS METHOD |
| 9 | 0.0000090255 | ABSORBENCY RATIO//PEAK HOMOGENEITY//PEAK PURITY |
| 10 | 0.0000085068 | CLASSICAL ESTIMATOR//INVERSE ESTIMATOR//ERRORS IN BOTH AXES |