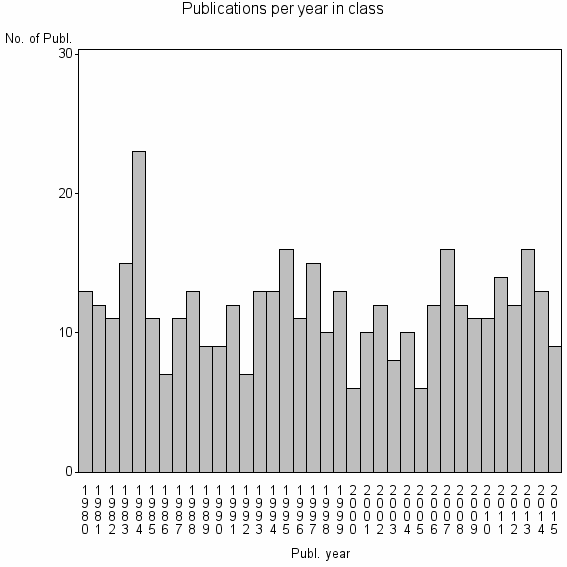
Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
| 19867 | 422 | 15.9 | 36% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 1386 | 7667 | SPRAY DRYING//FREEZE DRYING//LYOPHILIZATION |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | DRUG STABILITY STUDY | Author keyword | 4 | 75% | 1% | 3 |
| 2 | EXPIRATION DATING PERIOD | Author keyword | 3 | 57% | 1% | 4 |
| 3 | BRACKETING DESIGN | Author keyword | 3 | 100% | 1% | 3 |
| 4 | RETEST PERIOD | Author keyword | 3 | 100% | 1% | 3 |
| 5 | HIGH TEMPERATURE HIGH HUMIDITY | Author keyword | 2 | 67% | 0% | 2 |
| 6 | INSULIN HUMAN ISOPHANE | Author keyword | 2 | 67% | 0% | 2 |
| 7 | MATRIX DESIGNS | Author keyword | 2 | 67% | 0% | 2 |
| 8 | DEV US | Address | 1 | 100% | 0% | 2 |
| 9 | EARLY STAGE DEVELOPMENT | Author keyword | 1 | 100% | 0% | 2 |
| 10 | HEAT SEAL LACQUER | Author keyword | 1 | 100% | 0% | 2 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | DRUG STABILITY STUDY | 4 | 75% | 1% | 3 | Search DRUG+STABILITY+STUDY | Search DRUG+STABILITY+STUDY |
| 2 | EXPIRATION DATING PERIOD | 3 | 57% | 1% | 4 | Search EXPIRATION+DATING+PERIOD | Search EXPIRATION+DATING+PERIOD |
| 3 | BRACKETING DESIGN | 3 | 100% | 1% | 3 | Search BRACKETING+DESIGN | Search BRACKETING+DESIGN |
| 4 | RETEST PERIOD | 3 | 100% | 1% | 3 | Search RETEST+PERIOD | Search RETEST+PERIOD |
| 5 | HIGH TEMPERATURE HIGH HUMIDITY | 2 | 67% | 0% | 2 | Search HIGH+TEMPERATURE+HIGH+HUMIDITY | Search HIGH+TEMPERATURE+HIGH+HUMIDITY |
| 6 | INSULIN HUMAN ISOPHANE | 2 | 67% | 0% | 2 | Search INSULIN+HUMAN+ISOPHANE | Search INSULIN+HUMAN+ISOPHANE |
| 7 | MATRIX DESIGNS | 2 | 67% | 0% | 2 | Search MATRIX+DESIGNS | Search MATRIX+DESIGNS |
| 8 | EARLY STAGE DEVELOPMENT | 1 | 100% | 0% | 2 | Search EARLY+STAGE+DEVELOPMENT | Search EARLY+STAGE+DEVELOPMENT |
| 9 | HEAT SEAL LACQUER | 1 | 100% | 0% | 2 | Search HEAT+SEAL+LACQUER | Search HEAT+SEAL+LACQUER |
| 10 | MATRIXING DESIGN | 1 | 100% | 0% | 2 | Search MATRIXING+DESIGN | Search MATRIXING+DESIGN |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | DRUG STABILITY | 5 | 50% | 2% | 7 |
| 2 | PRODUCT STABILITY | 2 | 67% | 0% | 2 |
| 3 | POOLING BATCHES | 2 | 43% | 1% | 3 |
| 4 | ACCELERATED STABILITY | 1 | 50% | 0% | 2 |
| 5 | GELATIN CAPSULE BRITTLENESS | 1 | 100% | 0% | 2 |
| 6 | STABILITY DATA | 1 | 100% | 0% | 2 |
| 7 | DISSOLUTION STABILITY | 1 | 33% | 1% | 3 |
| 8 | DISINTEGRATING AGENTS | 1 | 50% | 0% | 1 |
| 9 | STABILITY GUIDELINES | 1 | 50% | 0% | 1 |
| 10 | CHLORIDE INJECTION | 1 | 20% | 1% | 3 |
Journals |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| The Application of the Accelerated Stability Assessment Program (ASAP) to Quality by Design (QbD) for Drug Product Stability | 2011 | 9 | 10 | 60% |
| Stability Testing of Pharmaceutical Products - Comparison of Stability Testing Guidelines | 2010 | 11 | 21 | 62% |
| CURRENT PERSPECTIVES ON THE DISSOLUTION STABILITY OF SOLID ORAL DOSAGE FORMS | 1993 | 39 | 47 | 43% |
| Package Selection for Moisture Protection for Solid, Oral Drug Products | 2010 | 17 | 40 | 25% |
| Integration of temperature-controlled requirements into pharmacy practice | 2009 | 1 | 6 | 100% |
| Accelerated aging: Prediction of chemical stability of pharmaceuticals | 2005 | 97 | 81 | 17% |
| Drugs and drug administration in extreme environments | 2006 | 17 | 25 | 24% |
| Data analysis in stability studies of biopharmaceutical drugs with isothermal and non-isothermal assays | 2011 | 1 | 36 | 47% |
| An overview of stability studies | 1996 | 0 | 6 | 100% |
| CURRENT ISSUES IN REGULATORY REQUIREMENTS OF DRUG STABILITY | 1995 | 1 | 5 | 80% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | DEV US | 1 | 100% | 0.5% | 2 |
| 2 | ORAL LIQUID FORMULAT | 1 | 100% | 0.5% | 2 |
| 3 | BIOMET DATA MANAGEMENT | 1 | 50% | 0.2% | 1 |
| 4 | DRUG DELIVERY NANO PHARMACEUT | 1 | 50% | 0.2% | 1 |
| 5 | LENOR ZEEH PHARMACEUT EXPT STN | 1 | 50% | 0.2% | 1 |
| 6 | SK HUMAN AD TAT COUNTERMEASU | 1 | 50% | 0.2% | 1 |
| 7 | STAT SCI UNIT | 1 | 50% | 0.2% | 1 |
| 8 | ABBOTT | 0 | 33% | 0.2% | 1 |
| 9 | DEV ITABASHI KU | 0 | 33% | 0.2% | 1 |
| 10 | NONCLIN STAT GRP | 0 | 33% | 0.2% | 1 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000230425 | GRP PROPRIETES PSYCHOSENSORIELLES MAT//DISCOLORATION KINETICS//APPEARANCE TESTING |
| 2 | 0.0000162267 | COMPATIBILITY STUDIES//THERMAL ANAL ENVIRONM PROBLEMS//LUDEM |
| 3 | 0.0000078974 | SUCCINYLMONOCHOLINE//PANCURONIUM BROMIDE//AGED EXHUMATION TISSUES |
| 4 | 0.0000075821 | TRIMETHOPRIM TMP//PEDIATRIC OSTEOMYELITIS//EA 4066 |
| 5 | 0.0000072086 | S3RI//STAT OPERAT TECHNOL//BUSINESS INFORMAT ANALYT |
| 6 | 0.0000061954 | CAKING//ANTICAKING AGENTS//DELIQUESCENCE LOWERING |
| 7 | 0.0000058637 | SUBSTANDARD DRUGS//ONLINE PHARMACIES//ONLINE PHARMACY |
| 8 | 0.0000052380 | CARISSA EDULIS//CARISSA OPACA//CARISSA SPINARUM |
| 9 | 0.0000051977 | ROLLER COMPACTION//DRY GRANULATION//HECKEL PLOT |
| 10 | 0.0000050881 | AMORPHOUS CONTENT//ISOTHERMAL MICROCALORIMETRY//SORPTION CALORIMETRY |