Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
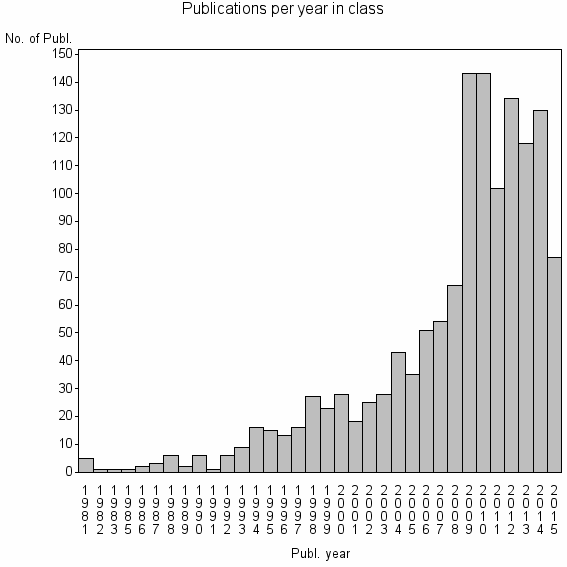
| 6946 | 1349 | 28.1 | 46% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 1443 | 7316 | EQ 5D//HEALTH TECHNOLOGY ASSESSMENT//INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | HEALTH TECHNOLOGY ASSESSMENT | Author keyword | 152 | 44% | 19% | 263 |
| 2 | INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE | Journal | 102 | 26% | 25% | 342 |
| 3 | COVERAGE WITH EVIDENCE DEVELOPMENT | Author keyword | 30 | 84% | 1% | 16 |
| 4 | FOURTH HURDLE | Author keyword | 18 | 83% | 1% | 10 |
| 5 | HTA | Author keyword | 14 | 35% | 2% | 32 |
| 6 | HOSPITAL BASED HTA | Author keyword | 11 | 100% | 0% | 6 |
| 7 | TECHNOLOGY ASSESSMENT | Author keyword | 10 | 11% | 7% | 88 |
| 8 | HORIZON SCANNING | Author keyword | 8 | 38% | 1% | 16 |
| 9 | JOURNAL OF COMPARATIVE EFFECTIVENESS RESEARCH | Journal | 7 | 18% | 2% | 33 |
| 10 | EUNETHTA | Author keyword | 7 | 67% | 0% | 6 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | HEALTH TECHNOLOGY ASSESSMENT | 152 | 44% | 19% | 263 | Search HEALTH+TECHNOLOGY+ASSESSMENT | Search HEALTH+TECHNOLOGY+ASSESSMENT |
| 2 | COVERAGE WITH EVIDENCE DEVELOPMENT | 30 | 84% | 1% | 16 | Search COVERAGE+WITH+EVIDENCE+DEVELOPMENT | Search COVERAGE+WITH+EVIDENCE+DEVELOPMENT |
| 3 | FOURTH HURDLE | 18 | 83% | 1% | 10 | Search FOURTH+HURDLE | Search FOURTH+HURDLE |
| 4 | HTA | 14 | 35% | 2% | 32 | Search HTA | Search HTA |
| 5 | HOSPITAL BASED HTA | 11 | 100% | 0% | 6 | Search HOSPITAL+BASED+HTA | Search HOSPITAL+BASED+HTA |
| 6 | TECHNOLOGY ASSESSMENT | 10 | 11% | 7% | 88 | Search TECHNOLOGY+ASSESSMENT | Search TECHNOLOGY+ASSESSMENT |
| 7 | HORIZON SCANNING | 8 | 38% | 1% | 16 | Search HORIZON+SCANNING | Search HORIZON+SCANNING |
| 8 | EUNETHTA | 7 | 67% | 0% | 6 | Search EUNETHTA | Search EUNETHTA |
| 9 | SCOTTISH MEDICINES CONSORTIUM | 6 | 80% | 0% | 4 | Search SCOTTISH+MEDICINES+CONSORTIUM | Search SCOTTISH+MEDICINES+CONSORTIUM |
| 10 | EARLY BENEFIT ASSESSMENT | 6 | 100% | 0% | 4 | Search EARLY+BENEFIT+ASSESSMENT | Search EARLY+BENEFIT+ASSESSMENT |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | HEALTH TECHNOLOGY ASSESSMENT | 24 | 30% | 5% | 69 |
| 2 | COVERAGE DECISIONS | 19 | 58% | 2% | 22 |
| 3 | NICE | 18 | 24% | 5% | 63 |
| 4 | 4TH HURDLE | 17 | 79% | 1% | 11 |
| 5 | EFFECTIVENESS INFORMATION | 15 | 88% | 1% | 7 |
| 6 | REIMBURSEMENT SCHEMES | 14 | 100% | 1% | 7 |
| 7 | CARE TECHNOLOGY | 13 | 80% | 1% | 8 |
| 8 | OFT | 12 | 86% | 0% | 6 |
| 9 | CLINICAL EXCELLENCE | 11 | 36% | 2% | 25 |
| 10 | EMERGING HEALTH TECHNOLOGIES | 10 | 73% | 1% | 8 |
Journals |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | INTERNATIONAL JOURNAL OF TECHNOLOGY ASSESSMENT IN HEALTH CARE | 102 | 26% | 25% | 342 |
| 2 | JOURNAL OF COMPARATIVE EFFECTIVENESS RESEARCH | 7 | 18% | 2% | 33 |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| European perspective on the costs and cost-effectiveness of cancer therapies | 2007 | 63 | 3 | 100% |
| Linking payment to health outcomes: A taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers | 2010 | 45 | 10 | 70% |
| Role of patient and public participation in health technology assessment and coverage decisions | 2011 | 21 | 23 | 65% |
| Harmonization of reimbursement and regulatory approval processes: a systematic review of international experiences | 2013 | 6 | 13 | 92% |
| Rapid reviews versus full systematic reviews: An inventory of current methods and practice in health technology assessment | 2008 | 35 | 6 | 83% |
| Methodological guidance documents for evaluation of ethical considerations in health technology assessment: a systematic review | 2014 | 3 | 29 | 69% |
| Cost, Coverage, and Comparative Effectiveness Research: The Critical Issues for Oncology | 2012 | 11 | 12 | 75% |
| Priority setting for health technology assessments: A systematic review of current practical approaches | 2007 | 36 | 16 | 94% |
| When Does NICE Recommend the Use of Health Technologies Within a Programme of Evidence Development? | 2013 | 4 | 12 | 100% |
| Common Drug Review Recommendations An Evidence Base for Expectations? | 2012 | 10 | 15 | 73% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | EVALUAT VALUE RISK HLTH | 5 | 21% | 1.7% | 23 |
| 2 | COORDINATING HLTH TECHNOL ASSESSMENT | 4 | 50% | 0.4% | 6 |
| 3 | MED TECHNOL POLICY | 3 | 57% | 0.3% | 4 |
| 4 | ABORATING HEALTHY PUBL POLICY | 3 | 100% | 0.2% | 3 |
| 5 | DESIGN SERV SE | 3 | 100% | 0.2% | 3 |
| 6 | VITORIA GASTEIZ | 3 | 100% | 0.2% | 3 |
| 7 | SBU | 3 | 40% | 0.4% | 6 |
| 8 | DANISH HLTH TECHNOL ASSESSMENT | 3 | 60% | 0.2% | 3 |
| 9 | PLANIFICAC SANITARIA | 3 | 60% | 0.2% | 3 |
| 10 | CANC RHEIN MAIN | 2 | 67% | 0.1% | 2 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000178563 | PRIORITY SETTING//ACCOUNTABILITY FOR REASONABLENESS//RATIONING |
| 2 | 0.0000154744 | COST EFFECTIVENESS ANALYSIS//BRIGHAM WOMENS FAULKNER NEUROL//OPTIMAL TRIAL DESIGN |
| 3 | 0.0000147318 | NUCLEAR VLIMATA//NUCLEAR VLIMA//CALCIUM ACTIVATED NEUTRAL PROTEINASE |
| 4 | 0.0000106608 | CARDIOVASC METABOL SERV//FORMULARY DEV//SPECIALTY DRUGS |
| 5 | 0.0000089595 | ORPHAN DRUGS//RARE DISEASES//OFF ORPHAN PROD DEV |
| 6 | 0.0000080916 | GENERIC SUBSTITUTION//GENERIC DRUGS//REFERENCE PRICING |
| 7 | 0.0000078156 | PATIENT AND PUBLIC INVOLVEMENT//HEALTH EXPECTATIONS//USER INVOLVEMENT |
| 8 | 0.0000074557 | SOCIAL IND POLICY GRP//DRUG DELIVERY PRODUCT//MEDICINE UTILIZATION |
| 9 | 0.0000059454 | RESEARCH PAYBACK//RAND EUROPE//KINGS POLICY |
| 10 | 0.0000058546 | R ID LEARNING PROJECT//PATIENT AND FAMILY SATISFACTION//FOOD BELIEFS AND PRACTICES |