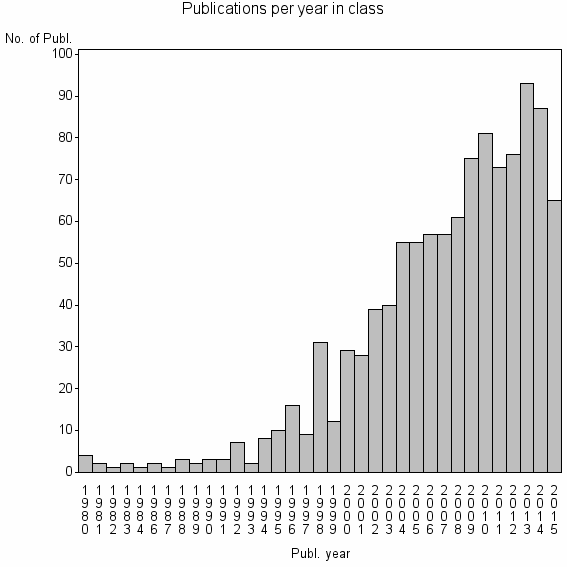
Class information for: |
Basic class information |
| ID | Publications | Average number of references |
Avg. shr. active ref. in WoS |
|---|---|---|---|
| 9404 | 1090 | 31.5 | 47% |
Classes in level above (level 2) |
| ID, lev. above |
Publications | Label for level above |
|---|---|---|
| 991 | 9999 | INFORMED CONSENT//RESEARCH ETHICS//MEDICAL ETHICS |
Terms with highest relevance score |
| Rank | Term | Type of term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|---|
| 1 | EQUIPOISE | Author keyword | 11 | 33% | 3% | 28 |
| 2 | SHAM SURGERY | Author keyword | 10 | 54% | 1% | 13 |
| 3 | DEVELOPING WORLD BIOETHICS | Journal | 10 | 22% | 4% | 39 |
| 4 | FAIR BENEFITS | Author keyword | 6 | 80% | 0% | 4 |
| 5 | BMC MEDICAL ETHICS | Journal | 6 | 13% | 4% | 41 |
| 6 | INTERNATIONAL RESEARCH ETHICS | Author keyword | 6 | 100% | 0% | 4 |
| 7 | MICHAEL KIRBY PUBL HLTH HUMAN RIGHTS | Address | 6 | 50% | 1% | 8 |
| 8 | CLIN BIOETH | Address | 5 | 14% | 3% | 36 |
| 9 | CLINICAL EQUIPOISE | Author keyword | 5 | 41% | 1% | 9 |
| 10 | HAVEG | Address | 4 | 67% | 0% | 4 |
Web of Science journal categories |
Author Key Words |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | EQUIPOISE | 11 | 33% | 3% | 28 | Search EQUIPOISE | Search EQUIPOISE |
| 2 | SHAM SURGERY | 10 | 54% | 1% | 13 | Search SHAM+SURGERY | Search SHAM+SURGERY |
| 3 | FAIR BENEFITS | 6 | 80% | 0% | 4 | Search FAIR+BENEFITS | Search FAIR+BENEFITS |
| 4 | INTERNATIONAL RESEARCH ETHICS | 6 | 100% | 0% | 4 | Search INTERNATIONAL+RESEARCH+ETHICS | Search INTERNATIONAL+RESEARCH+ETHICS |
| 5 | CLINICAL EQUIPOISE | 5 | 41% | 1% | 9 | Search CLINICAL+EQUIPOISE | Search CLINICAL+EQUIPOISE |
| 6 | HEALTH CAPABILITY PARADIGM | 3 | 100% | 0% | 3 | Search HEALTH+CAPABILITY+PARADIGM | Search HEALTH+CAPABILITY+PARADIGM |
| 7 | BELMONT REPORT | 3 | 35% | 1% | 7 | Search BELMONT+REPORT | Search BELMONT+REPORT |
| 8 | COMMUNITY ADVISORY BOARDS | 3 | 50% | 0% | 4 | Search COMMUNITY+ADVISORY+BOARDS | Search COMMUNITY+ADVISORY+BOARDS |
| 9 | INTERNATIONAL HEALTH RESEARCH | 3 | 60% | 0% | 3 | Search INTERNATIONAL+HEALTH+RESEARCH | Search INTERNATIONAL+HEALTH+RESEARCH |
| 10 | THERAPEUTIC OBLIGATION | 3 | 60% | 0% | 3 | Search THERAPEUTIC+OBLIGATION | Search THERAPEUTIC+OBLIGATION |
Key Words Plus |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | EQUIPOISE | 22 | 31% | 6% | 61 |
| 2 | ANCILLARY CARE | 15 | 71% | 1% | 12 |
| 3 | INTERNATIONAL RESEARCH | 15 | 50% | 2% | 21 |
| 4 | RESEARCH ETHICS | 13 | 28% | 4% | 39 |
| 5 | MEDICAL RESEARCHERS | 11 | 47% | 2% | 17 |
| 6 | POSTTRIAL ACCESS | 11 | 100% | 1% | 6 |
| 7 | TRANSMISSION PREVENTION TRIALS | 10 | 73% | 1% | 8 |
| 8 | PROTECTING COMMUNITIES | 9 | 34% | 2% | 21 |
| 9 | VACCINE TRIAL | 9 | 31% | 2% | 24 |
| 10 | THERAPEUTIC MISCONCEPTION | 9 | 18% | 4% | 44 |
Journals |
| Rank | Web of Science journal category | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | DEVELOPING WORLD BIOETHICS | 10 | 22% | 4% | 39 |
| 2 | BMC MEDICAL ETHICS | 6 | 13% | 4% | 41 |
Reviews |
| Title | Publ. year | Cit. | Active references |
% act. ref. to same field |
|---|---|---|---|---|
| Community engagement strategies for genomic studies in Africa: a review of the literature | 2015 | 2 | 41 | 66% |
| The quality of informed consent: mapping the landscape. A review of empirical data from developing and developed countries | 2012 | 20 | 46 | 41% |
| Towards a framework for community engagement in global health research | 2010 | 27 | 12 | 58% |
| Reasons Why Post-Trial Access to Trial Drugs Should, or Need not be Ensured to Research Participants: A Systematic Review | 2011 | 10 | 30 | 83% |
| What makes clinical research ethical? | 2000 | 700 | 29 | 28% |
| Ethics in clinical research: Need for assessing comprehension of informed consent form? | 2011 | 4 | 2 | 100% |
| Treatment success in pragmatic randomised controlled trials: a review of trials funded by the UK Health Technology Assessment programme | 2011 | 5 | 11 | 73% |
| Informed consent in international health research | 2006 | 29 | 48 | 50% |
| Conduct of clinical trials in developing countries: a perspective | 2009 | 7 | 11 | 64% |
| Optimism bias leads to inconclusive results-an empirical study | 2011 | 9 | 12 | 42% |
Address terms |
| Rank | Address term | Relevance score (tfidf) |
Class's shr. of term's tot. occurrences |
Shr. of publ. in class containing term |
Num. of publ. in class |
|---|---|---|---|---|---|
| 1 | MICHAEL KIRBY PUBL HLTH HUMAN RIGHTS | 6 | 50% | 0.7% | 8 |
| 2 | CLIN BIOETH | 5 | 14% | 3.3% | 36 |
| 3 | HAVEG | 4 | 67% | 0.4% | 4 |
| 4 | ETHOX | 3 | 13% | 2.1% | 23 |
| 5 | ADVANCEMENT PL ETH | 2 | 67% | 0.2% | 2 |
| 6 | CEBESA | 2 | 67% | 0.2% | 2 |
| 7 | CLIN UNIT NANORO | 2 | 67% | 0.2% | 2 |
| 8 | ETH LAW HUMAN RIGHTS ABORATING | 2 | 67% | 0.2% | 2 |
| 9 | ETH SOCIAL CULTURAL RISK | 2 | 67% | 0.2% | 2 |
| 10 | HLTH OUTCOME BEHAV | 2 | 67% | 0.2% | 2 |
Related classes at same level (level 1) |
| Rank | Relatedness score | Related classes |
|---|---|---|
| 1 | 0.0000198332 | SERV PSYCHIAT MENTAL HLTH//SYMPTOMATIC VOLUNTEERS//UNDUE INDUCEMENT |
| 2 | 0.0000196603 | MEDICAL WAR CRIMES//UNIVERSAL DECLARATION ON BIOETHICS AND HUMAN RIGHTS//LEO ALEXANDER |
| 3 | 0.0000187547 | RESEARCH ETHICS COMMITTEES//INSTITUTIONAL REVIEW BOARD//IRB |
| 4 | 0.0000167439 | HIV VACCINE TRIALS//HIV VACCINES//WILLINGNESS TO PARTICIPATE |
| 5 | 0.0000167069 | ASSENT//RESEARCH PARTICIPATION DECISION MAKING//NON THERAPEUTIC RESEARCH |
| 6 | 0.0000131041 | PATIENT RECRUITMENT//MINORITY RECRUITMENT//TRIAL PARTICIPATION |
| 7 | 0.0000129400 | RESEARCH CAPACITY STRENGTHENING//HEALTH RESEARCH INSTITUTIONS//HEALTH RESEARCH POLICY AND SYSTEMS |
| 8 | 0.0000113733 | EMERGENCY RESEARCH//EXCEPTION FROM INFORMED CONSENT//WAIVER OF CONSENT |
| 9 | 0.0000088897 | PUBLIC HEALTH ETHICS//PRACT ETH PUBL LIFE//PROGRAM LAW PUBL HLTH |
| 10 | 0.0000088469 | BIOBANKS//RETURN OF RESULTS//BIOBANK |