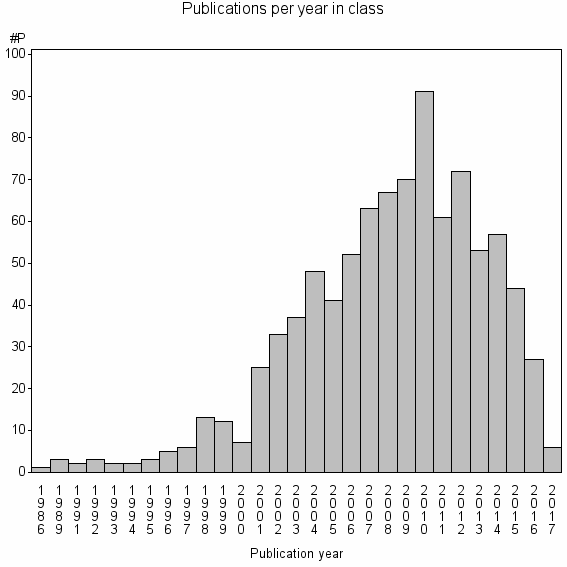
Class information for: |
Basic class information |
| Class id | #P | Avg. number of references |
Database coverage of references |
|---|---|---|---|
| 12424 | 906 | 49.3 | 90% |
Hierarchy of classes |
The table includes all classes above and classes immediately below the current class. |
| Cluster id | Level | Cluster label | #P |
|---|---|---|---|
| 7 | 4 | INFECTIOUS DISEASES//MICROBIOLOGY//VIROLOGY | 1353914 |
| 29 | 3 | HIV//VIROLOGY//HIV 1 | 108954 |
| 10 | 2 | AIDS RESEARCH AND HUMAN RETROVIRUSES//VIROLOGY//HIV 1 | 39664 |
| 12424 | 1 | HIV VACCINE//MOL MUCOSAL VACCINE IMMUNOL GRP//HIV VACCINE TRIALS NETWORK | 906 |
Terms with highest relevance score |
| rank | Term | termType | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|---|
| 1 | HIV VACCINE | authKW | 434231 | 7% | 20% | 66 |
| 2 | MOL MUCOSAL VACCINE IMMUNOL GRP | address | 209984 | 1% | 69% | 9 |
| 3 | HIV VACCINE TRIALS NETWORK | address | 176054 | 2% | 33% | 16 |
| 4 | VACCINE | address | 148101 | 11% | 4% | 104 |
| 5 | VACCINE BRANCH | address | 145822 | 4% | 12% | 35 |
| 6 | STEP TRIAL | authKW | 140423 | 1% | 83% | 5 |
| 7 | MOL IMMUNOGENET VACCINE SECT | address | 137156 | 2% | 24% | 17 |
| 8 | PRIME BOOST | authKW | 128586 | 3% | 14% | 27 |
| 9 | CHIMPANZEE ADENOVIRUS | authKW | 107845 | 0% | 80% | 4 |
| 10 | AD5 IMMUNITY | authKW | 101106 | 0% | 100% | 3 |
Web of Science journal categories |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | Virology | 15772 | 32% | 0% | 288 |
| 2 | Immunology | 13019 | 57% | 0% | 516 |
| 3 | Medicine, Research & Experimental | 4406 | 26% | 0% | 240 |
| 4 | Infectious Diseases | 3098 | 18% | 0% | 162 |
| 5 | Biotechnology & Applied Microbiology | 326 | 9% | 0% | 85 |
| 6 | Microbiology | 209 | 8% | 0% | 70 |
| 7 | Genetics & Heredity | 38 | 4% | 0% | 38 |
| 8 | Multidisciplinary Sciences | 17 | 1% | 0% | 9 |
| 9 | Biochemical Research Methods | 14 | 2% | 0% | 21 |
| 10 | Mathematical & Computational Biology | 5 | 1% | 0% | 7 |
Address terms |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | MOL MUCOSAL VACCINE IMMUNOL GRP | 209984 | 1% | 69% | 9 |
| 2 | HIV VACCINE TRIALS NETWORK | 176054 | 2% | 33% | 16 |
| 3 | VACCINE | 148101 | 11% | 4% | 104 |
| 4 | VACCINE BRANCH | 145822 | 4% | 12% | 35 |
| 5 | MOL IMMUNOGENET VACCINE SECT | 137156 | 2% | 24% | 17 |
| 6 | IAVI CORE | 89869 | 0% | 67% | 4 |
| 7 | KAVI | 75828 | 0% | 75% | 3 |
| 8 | MEDUNSA HIV CLIN UNIT | 75828 | 0% | 75% | 3 |
| 9 | CORE OPERAT | 67404 | 0% | 100% | 2 |
| 10 | VACCINE PHARMACEUT DEV | 67404 | 0% | 100% | 2 |
Journals |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
|---|---|---|---|---|---|
| 1 | VACCINE | 38756 | 16% | 1% | 144 |
| 2 | CURRENT OPINION IN HIV AND AIDS | 17866 | 2% | 3% | 21 |
| 3 | JOURNAL OF VIROLOGY | 9854 | 12% | 0% | 108 |
| 4 | EXPERT REVIEW OF VACCINES | 8749 | 2% | 1% | 18 |
| 5 | AIDS RESEARCH AND HUMAN RETROVIRUSES | 6018 | 3% | 1% | 31 |
| 6 | MUCOSAL IMMUNOLOGY | 3425 | 1% | 1% | 9 |
| 7 | CLINICAL AND VACCINE IMMUNOLOGY | 2489 | 2% | 1% | 14 |
| 8 | HUMAN VACCINES & IMMUNOTHERAPEUTICS | 2398 | 1% | 1% | 11 |
| 9 | CURRENT HIV RESEARCH | 2000 | 1% | 1% | 7 |
| 10 | VIRAL IMMUNOLOGY | 1875 | 1% | 1% | 8 |
Author Key Words |
| Rank | Term | Chi square | Shr. of publ. in class containing term |
Class's shr. of term's tot. occurrences |
#P with term in class |
LCSH search | Wikipedia search |
|---|---|---|---|---|---|---|---|
| 1 | HIV VACCINE | 434231 | 7% | 20% | 66 | Search HIV+VACCINE | Search HIV+VACCINE |
| 2 | STEP TRIAL | 140423 | 1% | 83% | 5 | Search STEP+TRIAL | Search STEP+TRIAL |
| 3 | PRIME BOOST | 128586 | 3% | 14% | 27 | Search PRIME+BOOST | Search PRIME+BOOST |
| 4 | CHIMPANZEE ADENOVIRUS | 107845 | 0% | 80% | 4 | Search CHIMPANZEE+ADENOVIRUS | Search CHIMPANZEE+ADENOVIRUS |
| 5 | AD5 IMMUNITY | 101106 | 0% | 100% | 3 | Search AD5+IMMUNITY | Search AD5+IMMUNITY |
| 6 | ADENOVIRUS 26 | 101106 | 0% | 100% | 3 | Search ADENOVIRUS+26 | Search ADENOVIRUS+26 |
| 7 | POXVIRUS VECTORS | 101100 | 1% | 50% | 6 | Search POXVIRUS+VECTORS | Search POXVIRUS+VECTORS |
| 8 | NYVAC | 77030 | 0% | 57% | 4 | Search NYVAC | Search NYVAC |
| 9 | RECOMBINANT MODIFIED VACCINIA VIRUS ANKARA | 75828 | 0% | 75% | 3 | Search RECOMBINANT+MODIFIED+VACCINIA+VIRUS+ANKARA | Search RECOMBINANT+MODIFIED+VACCINIA+VIRUS+ANKARA |
| 10 | MVA | 74250 | 2% | 12% | 18 | Search MVA | Search MVA |
Core articles |
The table includes core articles in the class. The following variables is taken into account for the relevance score of an article in a cluster c: (1) Number of references referring to publications in the class. (2) Share of total number of active references referring to publications in the class. (3) Age of the article. New articles get higher score than old articles. (4) Citation rate, normalized to year. |
| Rank | Reference | # ref. in cl. |
Shr. of ref. in cl. |
Citations |
|---|---|---|---|---|
| 1 | ONDONDO, BO , (2014) THE INFLUENCE OF DELIVERY VECTORS ON HIV VACCINE EFFICACY.FRONTIERS IN MICROBIOLOGY. VOL. 5. ISSUE . P. - | 95 | 33% | 11 |
| 2 | EXCLER, JL , TOMARAS, GD , RUSSELL, ND , (2013) NOVEL DIRECTIONS IN HIV-1 VACCINES REVEALED FROM CLINICAL TRIALS.CURRENT OPINION IN HIV AND AIDS. VOL. 8. ISSUE 5. P. 421-431 | 56 | 47% | 14 |
| 3 | GRAY, GE , MOODIE, Z , METCH, B , GILBERT, PB , BEKKER, LG , CHURCHYARD, G , NCHABELENG, M , MLISANA, K , LAHER, F , ROUX, S , ET AL (2014) RECOMBINANT ADENOVIRUS TYPE 5 HIV GAG/POL/NEF VACCINE IN SOUTH AFRICA: UNBLINDED, LONG-TERM FOLLOW-UP OF THE PHASE 2H HVTN 503/PHAMBILI STUDY.LANCET INFECTIOUS DISEASES. VOL. 14. ISSUE 5. P. 388-396 | 27 | 79% | 33 |
| 4 | GOMEZ, CE , PERDIGUERO, B , GARCIA-ARRIAZA, J , ESTEBAN, M , (2012) POXVIRUS VECTORS AS HIV/AIDS VACCINES IN HUMANS.HUMAN VACCINES & IMMUNOTHERAPEUTICS. VOL. 8. ISSUE 9. P. 1192-1207 | 54 | 49% | 14 |
| 5 | PARIS, RM , KIM, JH , ROBB, ML , MICHAEL, NL , (2010) PRIME-BOOST IMMUNIZATION WITH POXVIRUS OR ADENOVIRUS VECTORS AS A STRATEGY TO DEVELOP A PROTECTIVE VACCINE FOR HIV-1.EXPERT REVIEW OF VACCINES. VOL. 9. ISSUE 9. P. 1055-1069 | 58 | 45% | 39 |
| 6 | BADEN, LR , LIU, JY , LI, HL , JOHNSON, JA , WALSH, SR , KLEINJAN, JA , ENGELSON, BA , PETER, L , ABBINK, P , MILNER, DA , ET AL (2015) INDUCTION OF HIV-1-SPECIFIC MUCOSAL IMMUNE RESPONSES FOLLOWING INTRAMUSCULAR RECOMBINANT ADENOVIRUS SEROTYPE 26 HIV-1 VACCINATION OF HUMANS.JOURNAL OF INFECTIOUS DISEASES. VOL. 211. ISSUE 4. P. 518 -528 | 23 | 82% | 12 |
| 7 | ZHANG, SJ , HUANG, WX , ZHOU, XY , ZHAO, QQ , WANG, Q , JIA, B , (2013) SEROPREVALENCE OF NEUTRALIZING ANTIBODIES TO HUMAN ADENOVIRUSES TYPE-5 AND TYPE-26 AND CHIMPANZEE ADENOVIRUS TYPE-68 IN HEALTHY CHINESE ADULTS.JOURNAL OF MEDICAL VIROLOGY. VOL. 85. ISSUE 6. P. 1077-1084 | 31 | 63% | 12 |
| 8 | O'CONNELL, RJ , KIM, JH , COREY, L , MICHAEL, NL , (2012) HUMAN IMMUNODEFICIENCY VIRUS VACCINE TRIALS.COLD SPRING HARBOR PERSPECTIVES IN MEDICINE. VOL. 2. ISSUE 12. P. - | 55 | 35% | 26 |
| 9 | HAYES, P , GILMOUR, J , VON LIEVEN, A , GILL, D , CLARK, L , KOPYCINSKI, J , CHEESEMAN, H , CHUNG, A , ALTER, G , DALLY, L , ET AL (2013) SAFETY AND IMMUNOGENICITY OF DNA PRIME AND MODIFIED VACCINIA ANKARA VIRUS-HIV SUBTYPE C VACCINE BOOST IN HEALTHY ADULTS.CLINICAL AND VACCINE IMMUNOLOGY. VOL. 20. ISSUE 3. P. 397 -408 | 32 | 57% | 9 |
| 10 | SANTRA, S , SUN, Y , KORIOTH-SCHMITZ, B , FITZGERALD, J , CHARBONNEAU, C , SANTOS, G , SEAMAN, MS , RATCLIFFE, SJ , MONTEFLORI, DC , NABEL, GJ , ET AL (2009) HETEROLOGOUS PRIME/BOOST IMMUNIZATIONS OF RHESUS MONKEYS USING CHIMPANZEE ADENOVIRUS VECTORS.VACCINE. VOL. 27. ISSUE 42. P. 5837-5845 | 25 | 78% | 28 |
Classes with closest relation at Level 1 |