Research Profile
Main Research Interests
The general research interest of Sun group is to challenge the so called “Holy Grail” of artificial photosynthesis, including molecular solar cells and solar fuels, with the aim to accelerate fundamental research to drive a conceptual transition from fossil fuels to solar energy systems. For solar cells, the Sun group is interested in solid state solar cells with cheap molecular components such as organic dyes, perovskites and hole/electron transport materials; For solar fuels, the Sun group has been trying to create man-made devices with molecular components, such as visible light driven water splitting to generate hydrogen. They are interested in water oxidation catalysts and hydrogen production catalysts with either molecular complexes inspired from natural Photosystem II and Hydrogenases, or first raw transition metal oxides/hydroxides, interfacial studies of molecular components (such as dyes and catalysts) with nano-structured semiconductors (TiO2, NiO etc), assembly of molecular devices for total water splitting, reduction of CO2 to CO, HCOOH, MeOH or even polycarbonates by electrochemical and photoelectrochemical methods, nitrogen fixation, light conversion into heat at molecular level are also within the interests of Sun group. The success of these projects will have a great impact on our society by offering sustainable energy systems without the costs of environment, and provides us with endless energy sources in the future.
Professor Sun has published more than 600 papers in international peer-reviewed journals, including Science, Nature Chemistry, Acc. Chem. Res., PNAS, JACS, Angew. Chem., Energy Environ. Sci., Adv. Energy Mater. with total number of citations more than 45 000 and an H-index of 100.
On-going research projects
1. Dye-sensitized solar cells (DSSCs) based on organic chromophores
Design and synthesis of organic dyes for sensitization of n-type nanostructured semiconductors such as TiO2 and p-type nanostructured semiconductors such as NiO.
2. New hole transport materials for solid state dye-sensitized solar cells (sDSSCs)
Design and synthesis of new hole transport materials to replace the commonly used Spiro-OMeTAD for solid state dye-sensitized solar cells (sDSSCs) and perovskite sensitized solar cells.
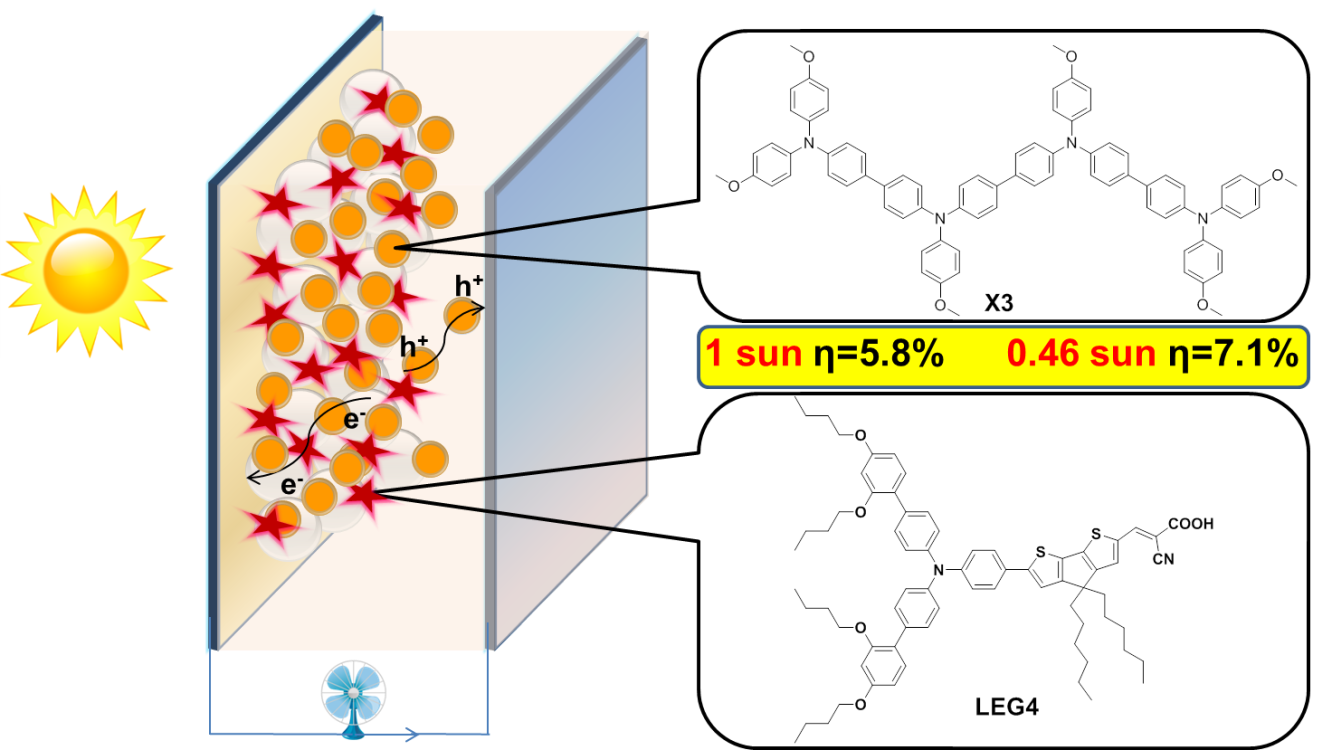
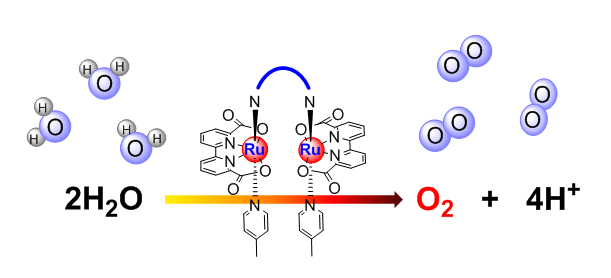
3. Bio(Photosystem II)-inspired molecular catalysts for water oxidation
Design, synthesis and catalytic properties of molecular catalysts based on transition metal complexes, such as Ru, Co, Cu and Fe complexes for chemical driven, electrochemical driven and visible light driven water oxidation. Mechanism study of O-O bond formation.
4. Molecular complexes for catalytic hydrogen production and nitrogen fixation
Synthesis and catalytic properties of FeS complexes as active site models of FeFe-hydrogenases and Nitrogenases for electrochemical and photo-electrochemical hydrogen generation and nitrogen fixation, coupled to water oxidation. Mechanism study of H-H bond formation and NN bond break.

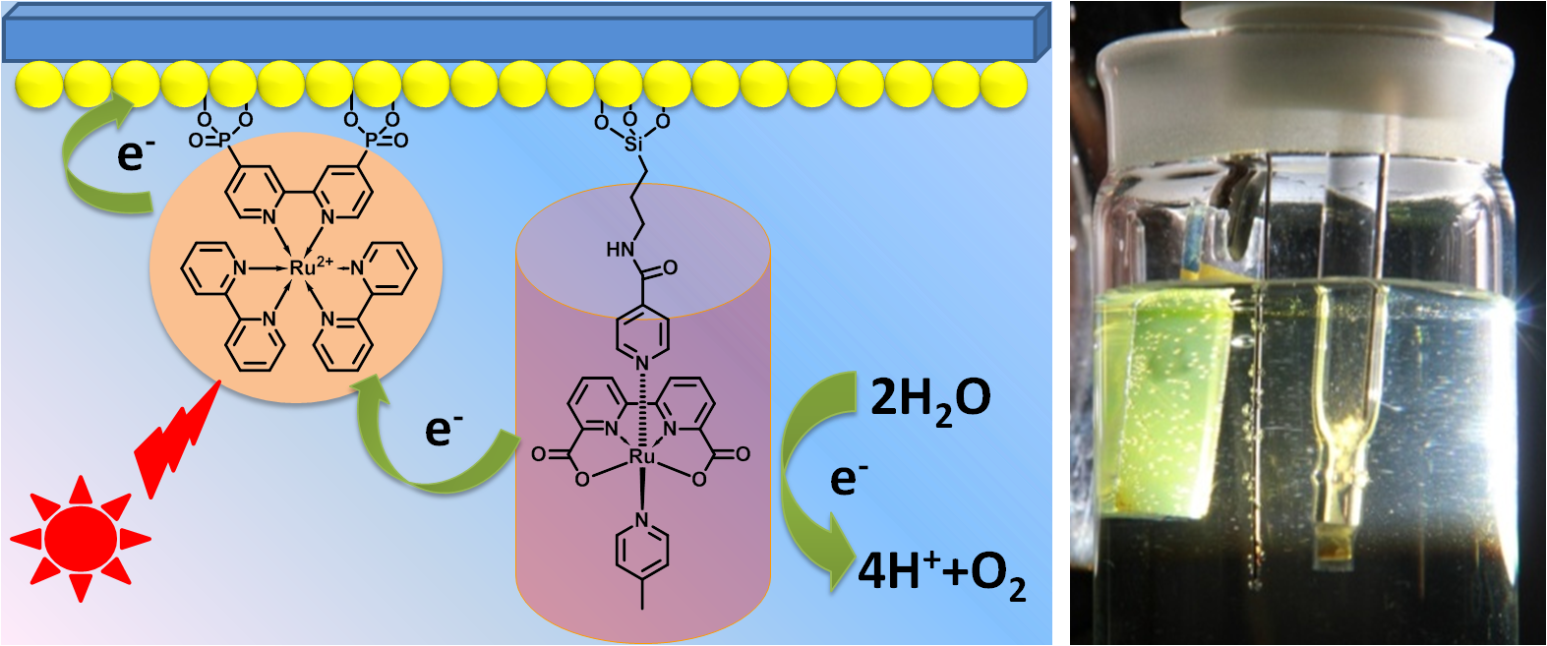
5. Functional devices based on molecular catalysts for light-driven total water splitting and nitrogen fixation
To immobilize the molecular water oxidation catalyst and molecular hydrogen generation catalyst/nitrogen reduction catalyst on respective anode and cathode electrode for electrochemical and photochemical total water splitting, and nitrogen fixation.
Brief account of previous research activities in Sun group
1. Photochemistry and photophysics of stilbene derivatives, using the techniques of laser flash photolysis and pulse radiolysis. For example, the protection of single or double strand DNA with ruthenium tris-bipyridyl complexes against OH• radical attack studied by pulse radiolysis technique.
2. Synthesis, characterization and photo-induced electron transfer studies of biomimetic model systems for the electron acceptor side of Photosystem II. For examples, tetraphenylporphyrins convalently linked to crown-ether quinones with different bridges (Angew. Chem. Int. Ed. Engl. 1994, 33, 2318-2320. Tetrahedron 1995, 51, 3535-3548. Solar Energy Mater. & Solar Cells 1995, 38, 91-110); photoinduced electron transfer and the subsequent charge separation studied in the nametic phase of liquid crystals (Magn. Reson. Chem. 1995, 33, Special Issue, S28-S33). This system could be used as light sensitive molecular probe to detect some metal ions such as Na+ and K+.
3. Synthesis, characterization and photo-induced electron transfer studies of biomimetic systems for the electron donor side of Photosystem II. A group of ruthenium-manganese binuclear complexes and porphyrin-manganese complexes have been synthesized with variation of bridging ligands and distances between ruthenium and manganese (J. Am. Chem. Soc. 1997, 119, 6996-7004. J. Phys. Chem. A 1998, 102, 2512-2518. Biochim. Biophys. Acta 1998, 1365, 193-199. Eur. J. Inorg. Chem. 2001, 1019-1029. Inorg. Chem. 2002, 41, 1534-1544).
4. Ru-complex covalently linked to L-tyrosine has been designed, synthesized and characterized. Photo-induced electron transfer studies showed that tyrosyl radical was generated in a similar way to the TyrosineZ radical in natural PS II (J. Am. Chem. Soc. 1997, 119, 10720-10725). It was also shown that the photo-generated tyrosyl radical could oxidize a Mn(III,III) dimer intermolecularly, resulting in the formation of high valence Mn(III,IV) complex (J. Am. Chem. Soc. 1999, 121, 89-96).
5. It was found that hydrogen bonded tyrosine strongly promoted the electron transfer from the tyrosine to the photo-generated Ru(III), closely mimicking the natural system (J. Am. Chem. Soc. 1999, 121, 6834-6842. J. Am. Chem. Soc. 2000, 122, 3932-3936) of PSII.
6. It was the first time that a Mn dimer has been covalently linked to the Ru complex, and a fast intramolecular electron transfer from the Mn(II,II) dimer to the photogenerated Ru(III) has been observed (J. Inorg. Biochem. 2000, 78, 15-22. Spectrochimica Acta A, 2001, 37, 2145-2160. J. Inorg. Biochem. 2002, 91, 159-172. Inorg. Chem. 2003, 42, 7502-7511).
7. Electron donor-acceptor triads based on benzoquinone acceptor linked to a photosensitive Ru(bpy)32+ complex have been synthesized and characterized. A fairly long lived (lifetime 80 ns) charge separated state with a high yield (>90 %) was observed in one of the supramolecular complexes after photo-induced electron transfer processes. Also, a series of ruthenium(II) polypyridine complexes linked to naphthalene diimide have been synthesized and characterized. Following excitation of the chromophore, a relatively long-lived charge-separated state was observed in one dyad supermolecule (Inorg. Chem. 2003, 42, 5173-5184. Inorg. Chem. 2003, 42, 2908-2918).
8. We have managed to synthesize phthalocyanines with different functional groups. Thus, a metal free and a Zn phthalocyanines with tetracarboxylate ethyl esters have been synthesized and characterized. By using a novel anchoring method, nanostructured TiO2 electrodes sensitized with these phthalocyanines have been made (Langmuir 2001, 17, 2743-2747). The performance of the solar cells based on these electrodes have been measured. To improve the efficiency of these systems, another phthalocyanine dye ZnPcTyr was designed and synthesized (J. Am. Chem. Soc. 2002, 124, 4922-4932). The synthesis of this molecule was focused on avoiding aggregation on the TiO2 oxide surface. We have managed to prepare phthalocyanine compounds with Ru(II) ion. Two substituted pyridines are coordinated to the Ru(II) ion as axial ligands (J. Porphyrins Phthalocyanines 2002, 6, 217-224). Absorption spectroscopy suggests that in ethanol solution of such a compound, no aggregations are found even at a high concentration.
9. A self-assembly monolayer of carotenoid and pheophytin on the surface of nanostructured TiO2 has been achieved. Photoinduced electron transfers of this system has been studied by femtosecond pump-probe technique (J. Am. Chem. Soc. 2002, 124, 13949-13957. J. Am. Chem. Soc. 2004, 126, 3066-3067), showing very fast electron injection from the S2 excited state.
10. As a biomimetic model of active site of Fe-only hydrogenases, a Fe2S2 dinuclear complex with a functional amino group has been synthesized (Chem. Eur. J. 2003, 9, 557-560). For the first time, a Fe2S2 dinuclear complex has been linked to a redox active species for the study of light induced electron transfer processes. The synthetic procedure and characterization of this complex has been described (Angew. Chem. Int. Ed. 2003, 42, 3285-3288). By using the electrochemical method, we have been successfully demonstrated the hydrogen production catalysed by Fe2S2 dinuclear complexes which are close models to the active site of Fe-only hydrogenase (Angew. Chem. Int. Ed. 2004, 43, 1006-1009. Chem. Eur. J. 2004, 10, 4474-4479. Angew. Chem. Int. Ed. 2004, 43, 3571-3574.).
11. Photo-induced electron transfer has been demonstrated in a three component system containing a electron donor, a photosensitizer and Fe2S2 active site models of Fe-only hydrogenases as catalysts (Chem. Commun. 2006, 305-307. Inorg. Chem. 2006, 45, 9169-9171. Inorg. Chem. 2007, 46, 1981-1991. Inorg. Chem. 2007, 46, 3813-3815). In the presence of acids, visible light driven hydrogen generation has been achieved with the three component systems using bioinspired FeS compounds as catalysts (Inorg. Chem. 2008, 47, 6948-6955. J. Phys. Chem. B 2008, 112, 8198-8202. Energy Environ. Sci. 2012, 5, 8220-8224), or Co and Ni complexes as the molecular catalysts (ChemSusChem 2012, 5, 2133-2138. Chem. Commun. 2013, 49, 9455-9457).
12. Supramolecular systems based on redox active Ru-tris-bipyridine complexes and CBs and CDs have been constructed and shown that they can functionalize as light driven molecular devices (Chem. Commun. 2006, 4195-4197. J. Phys. Chem. B. 2007, 111, 13357-13363. Chem. Commun. 2007, 4734-4736. J. Org. Chem. 2008, 73, 3775-3783, Eur. Org. Biomol. Chem. 2009, 7, 3605-3609, J. Org. Chem. 2009, 1163-1172, Chem. Commun. 2010, 46, 463-465. Chem. Eur. J. 2011, 17, 11604-11612).
13. Biomimetic active site models of FeFe-hydrogenases containing pendant amine ligands have been synthesized (Dalton Trans. 2009, 1919-1926). A iron hydride together with a proto on the pendant amine has been successfully isolated, and the isotope study showed that the hydride and the proton can be exchanged in solution, indicating the possible mechanism for H-H bond formation (Inorg. Chem. 2009, 48, 11551-11558. Chem. Commun. 2012, 48, 4450-4452).
14. A library of organic dyes have been designed and synthesized for applications in dye sensitized solar cells based on n-type semiconductors (TiO2) (Chem. Commun. 2006, 2245-2247. Chem. Mater. 2007, 19, 4007-4015. J. Phys. Chem. C. 2007, 111, 1853-1860. J. Org. Chem. 2007, 72, 9550-9556. J. Am. Chem. Soc. 2008, 130, 6259-6266). The efficiency of solar cells based on these organic dyes has reached more than 7%, and their photostability test gave satisfactory results (Angew. Chem. Int. Ed. 2009, 48, 1576-1580). Some of the organic dyes have also been applied to solid state solar cells with the efficiencies of more than 4.6% (J. Phys. Chem. C 2009, 113, 16816-16820. Synthetic Metals 2011, 161, 2280-2283. Adv. Funct. Mater. 2011, 21, 2944-2952. Phys. Chem. Chem. Phys. 2012, 14, 779-789. J. Phys. Chem. C 2012, 116, 18070-18078).
15. We have designed and synthesized organic chromophores for sensitization of p-type semiconductor NiO. A record high IPCE value has been achieved in solar cells based on these organic chromophores (J. Am. Chem. Soc. 2008, 130, 8570-8571; Adv. Mater., 2009, 21, 2993-2996. Adv. Mater. 2010, 22, 1759-1762. ChemSusChem 2013, 6, 1432-1437), providing the possibilities for new generation of solar cells with tandem systems. Visible light driven hydrogen production from a photoelectrochemical device based on molecular catalyst and organic dye-sensitized p-type nano NiO has been demonstrated recently (Chem. Commun. 2012, 48, 988-990).
16. The electronic and molecular structures of organic dyes on TiO2 interfaces for solar cell applications have been studied in a core level by photoelectron spectroscopy (J. Phys. Chem. C. 2007, 111, 8580-8586; Phys. Chem. Chem. Phys. 2010, 12, 1507-1517. Phys. Chem. Chem. Phys. 2011, 13, 3534-3546).
17. Several Quantum Dot/Rod Sensitized Solar Cells have been developed in our group with promising results (J. Am. Chem. Soc. 2011, 133, 8458-8460. Chem. Eur. J. 2011, 17, 6330-6333. ChemSusChem 2011, 4, 1741-1744. J. Mater. Chem. 2012, 22, 6032-6037), such as Solar Cells Sensitized with Type-II ZnSe-CdS Core/Shell Colloidal Quantum Dots (Chem. Commun. 2011, 47, 1536-1538). The utilization of colloidal up-conversion (UC) nanocrystals, such as NaYF4+Er+3, can significantly enhance the UC efficiency and improve the solar cell performance (J. Mater. Chem. 2012, 22, 16709-16713).
18. A broad range of Iodine-Free Redox Couples for Dye-Sensitized Solar Cells has been developed in our group to replace the classical iodide/tri-iodide redox couple, they all showed better performance (Angew. Chem. Int. Ed. 2010, 49, 7328-7331. J. Am. Chem. Soc. 2011, 133, 9413-9422. Chem. Commun. 2011, 47, 10124-10126. Chem. Eur. J. 2011, 17, 6330-6333. J. Mater. Chem. 2011, 21, 10592-10601. J. Mater. Chem. 2011, 21, 5573-5575. Energy Environ. Sci. 2012, 5, 9752-9755. Angew. Chem. Int. Ed. 2012, 51, 9896-9899. Phys. Chem. Chem. Phys. 2013, 15, 15146-15152. Nanoscale 2013, 5, 7963-7969).
19. As bioinspired(Photosystem II) water oxidation catalysts, a series of Ru complexes containing carboxylate ligands have been synthesized. It has been shown that these complexes can work as good catalysts for water oxidation driven by chemical oxidants, electrochemistry and visible light (Inorg. Chem. 2009, 48, 2717-2719; Inorg. Chem. 2010, 49, 209-215; Chem. Eur. J. 2010, 16, 4659-4668. Angew. Chem. Int. Ed. 2010, 49, 8934-8937. ChemSusChem 2011, 4, 238-244. Chem. Eur. J. 2011, 17, 9520-9528). During the catalytic water oxidation process, a very important intermediate, a high valent Ru(IV)Ru(IV) dimer with water molecules as seventh ligands coordinated to the Ru ions has been successfully isolated and structurally characterized by single crystal X-ray (J. Am. Chem. Soc. 2009, 131, 10397-10399). DTF calculation showed that mechanism for O-O bond formation goes via Ru(IV)-O radical coupling reaction (Angew. Chem. Int. Ed. 2010, 49, 1773-1777. Chem. Eur. J. 2011, 17, 8313-8317. J. Am. Chem. Soc. 2012, 134, 18868-18880. Inorg. Chem. 2013, 52, 7844-7852).
20. Light-driven water oxidation catalyzed by a supramolecular assemblies (Angew. Chem. Int. Ed. 2012, 51, 2417-2420. J. Catalysis. 2013, 306, 129-132) and in functional devices (Chem. Commun. 2010, 46, 7307-7309. Chem. Commun. 2012, 48,10025-10027) have been demonstrated. A proof of concept has been published by us (Chem. Commun. 2012, 48, 988-990) to immobilize a water reduction molecular catalyst on the surface of p-type semiconductor NiO as the cathode for light driven hydrogen generation.
21. A series of advanced water oxidation catalysts, based on Ru complexes and non-covalent pi-pi interactions of axial ligands have been invented. They have shown catalytic water oxidation by Ce(IV) with record high turnover frequency of more than 300 per second (Nature Chem. 2012, 4, 418-423. J. Am. Chem. Soc. 2012, 134, 18868-18880) and high stability with turnover numbers of more than 55 000 (PNAS 2012, 109, 15584-15588). Due to the nature of radical coupling mechanism of O-O bond formation, some bridged Ru dimer complexes have recently been shown very active towards water oxidation at even very low catalyst concentration, for example [10-8M] (Angew. Chem. Int. Ed. 2013, 52, 3398-3401).
22. A Fe2S2 compound bearing a pendant base as [FeFe]-hydrogenase mimic has been shown, in addition to the catalytic proton reduction to form hydrogen, to be able to oxidize the molecular hydrogen successfully as well under mild conditions. The detailed study on reaction mechanism of the H-H bond breaking has been performed by experiments and DFT calculation, revealing the involvement of μ-hydride species (J. Am. Chem. Soc. 2013, 135, 13688-13691).
23. Visible light driven total water splitting in a functional device with unprecedently high photocurrent density has been achieved using the nanostructured TiO2 as anode material, Ru(bpy)3 with anchoring groups as the photosensitizer and a Ru-bpa type complex as the molecular water oxidation catalyst. This device works well in aqueous solution with neutral pH (J. Am. Chem. Soc. 2013, 135, 4219-4222).
24. A series of new hole transport materials for the application in solid state dye-sensitized solar cells (sDSSCs) have been synthesized in our group. Some of the new materials can replace the commonly used Spiro-OMeTAD and show better solar cell performance (J. Am. Chem. Soc. 2013, 135, 7378-7385).
25. Highly efficient molecular catalysts for water oxidation
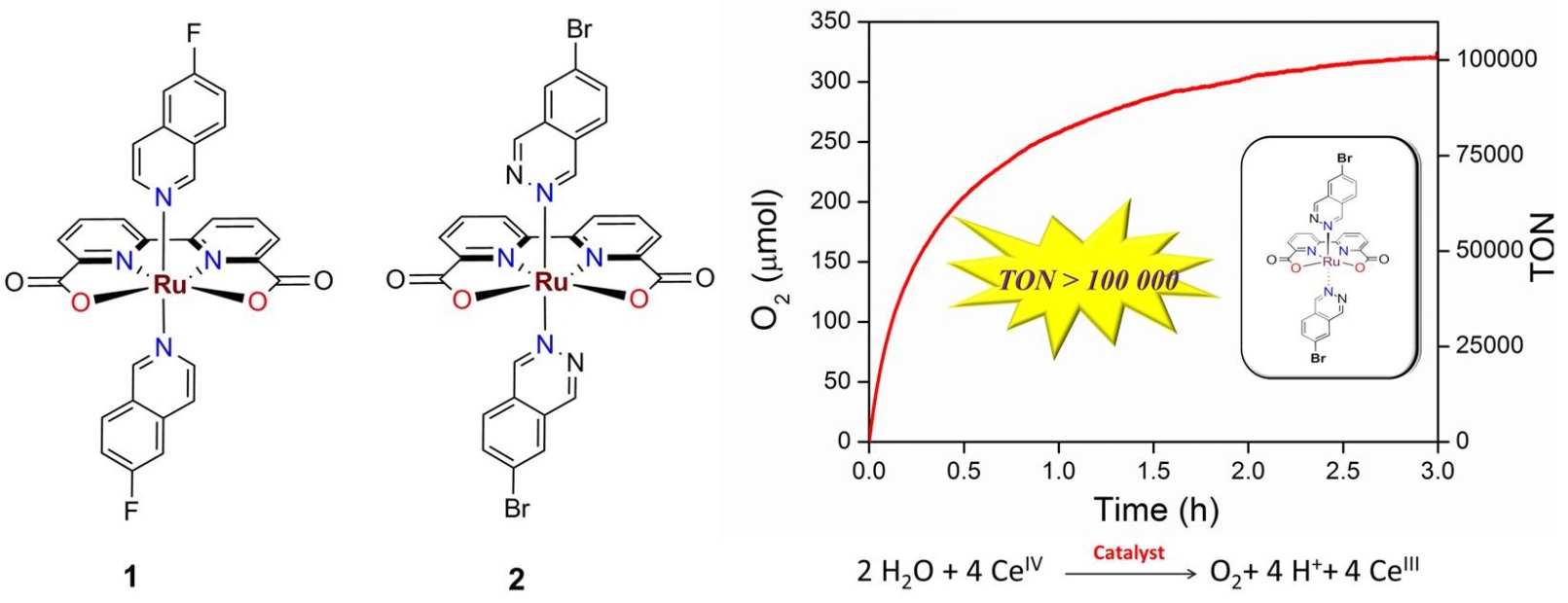
As one of the bottle neck problems of artificial photosynthesis and solar fuels production, development of highly efficient molecular catalysts for water oxidation and deep understanding of the O-O bond formation mechanisms are strongly desired presently. We have recently designed and synthesized 2 new Ru complexes (1 and 2) with 2,2’-bipyridine-6,6’-dicarboxylate (bda) ligand, and to our surprise all these two new complexes showed extremely high catalytic performance for water oxidation, an initial rate of more than 1000 turnover s-1 by complex 1, and a turnover number of more than 100 000 (in 3 hours) by complex 2 have been obtained by using CeIV as the chemical oxidant in pH 1.0 aqueous solutions [L. Wang et al, Chem. Commun. 2014, 50, 12947-12950]. These complexes are the most efficient and the most robust molecular water oxidation catalysts so far reported. Based on the electrochemical and kinetic studies, we found that the substitution groups on the axial ligands of these Ru complexes are very sensitive to the O-O bond formation via radical coupling pathway, providing some new insights into the water oxidation mechanism.
26. Immobilization of a molecular catalyst on carbon nanotubes for highly efficient electrocatalytic water oxidation
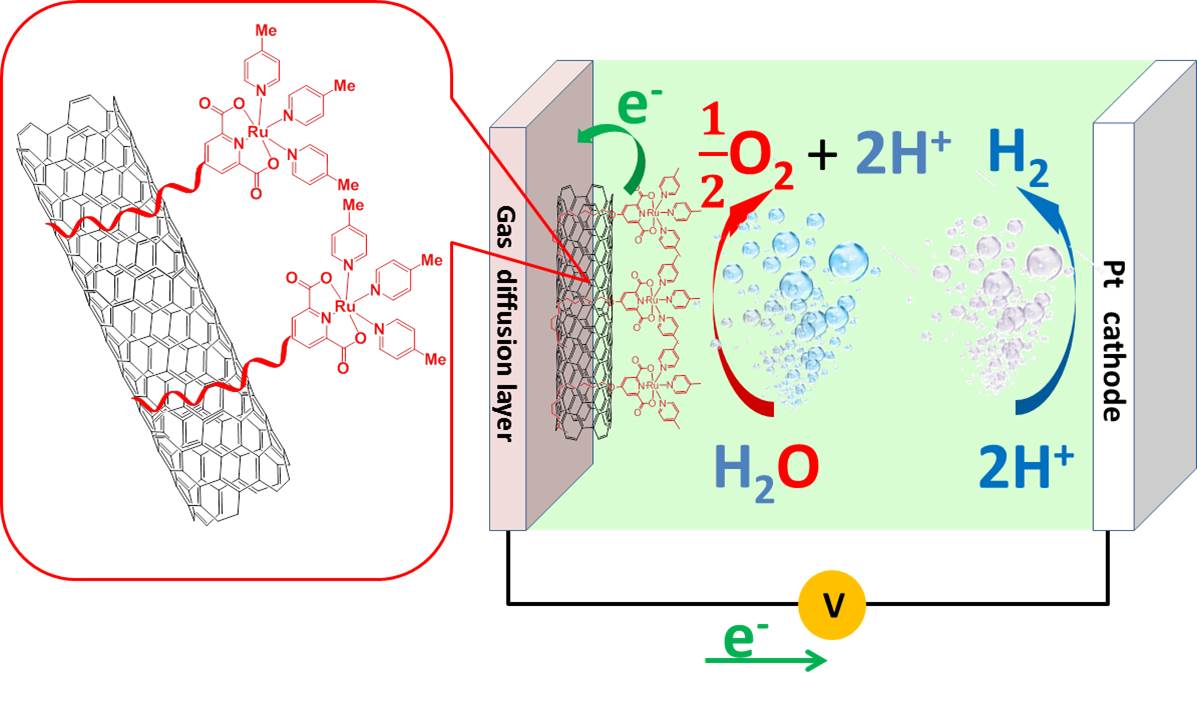
One of the major scientific challenges for the whole water splitting process is the lack of methods to immobilize water oxidation catalyst on electrode surface for potential device making. By using our efficient molecular water oxidation catalyst, we have now successfully demonstrated a universal method, by which molecular water oxidation catalysts can be firmly immobilized on conductive carbon nanotubes. A high turnover frequency (TOF) of 7.6 s−1 together with a high catalytic current density of 2.2 mA/cm2 was successfully obtained at an overpotential of 480 mV after 1 h electrolysis [F. Li et al, Chem. Commun. 2014, 50, 13948-13951], which is much higher than similar systems reported in literature.
27. High performance photoelectrochemical devices using molecular catalysts for light driven total water splitting
In collaboration with State Key Laboratory of Fine Chemicals, Dalian University of Technology (DUT) through the platform DUT-KTH Joint Education and Research Center on Molecular Devices, a binuclear and a mononuclear ruthenium water oxidation catalysts were synthesized and used in dye-sensitized photoelectrochemcal cells (PECs) for visible light driven water splitting. Two photoanodes TiO2(1+2) and TiO2(1+3) based on these two catalysts were assembled, respectively, in combination with a molecular photosensitizer 1 by using a co-adsorption method. The PEC device using the photoanode TiO2(1+2) exhibits a high photocurrent density of 1.1 mA/cm2 after 10 s illumination, which is twice as high as the corresponding value for the PEC with TiO2(1+3) as photoanode [L. Zhang et al, ChemSusChem 2014, 7, 2801-2804], and exhibits better performance on light driven water splitting. In a similar fashion, visible light driven water splitting has also been demonstrated in a PEC device where the photosensitizer and the water oxidation catalysts are self-assembled on the meso-porous TiO2 films, with a maximal incident photon to current conversion efficiency of 4.1% at ∼450 nm and a photocurrent density of ∼0.48 mA cm−2 [ACS Catalysis. 2014, 4, 2347-2350].
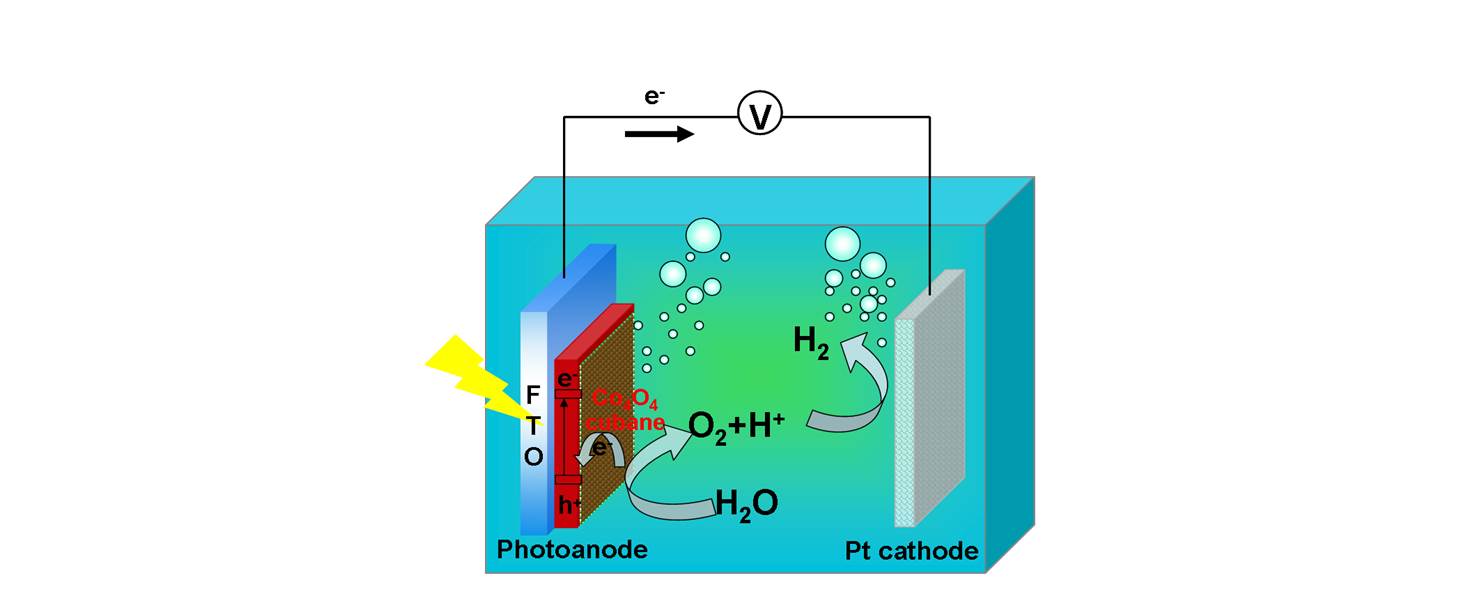
We have also shown that several cobalt−oxo cubanes are robust and efficient electrocatalysts for water oxidation as molecular mimics of the OEC in natural Photosystem II. The evidence obtained so far suggests the molecular identity of the catalytic active species on the electrode instead of cobalt oxides. Cobalt−oxo cubane was further integrated with α-Fe2O3 as a composite photoanode in a PEC device, which significantly shifts the onset potential for water oxidation and greatly enhances the photocurrent. To the best of our knowledge, this was the first time to achieve water oxidation by a combination of an inorganic semiconductor and a noble metal-free molecular catalyst [B. Zhang et al, ACS Catalysis. 2014, 4, 804-809].
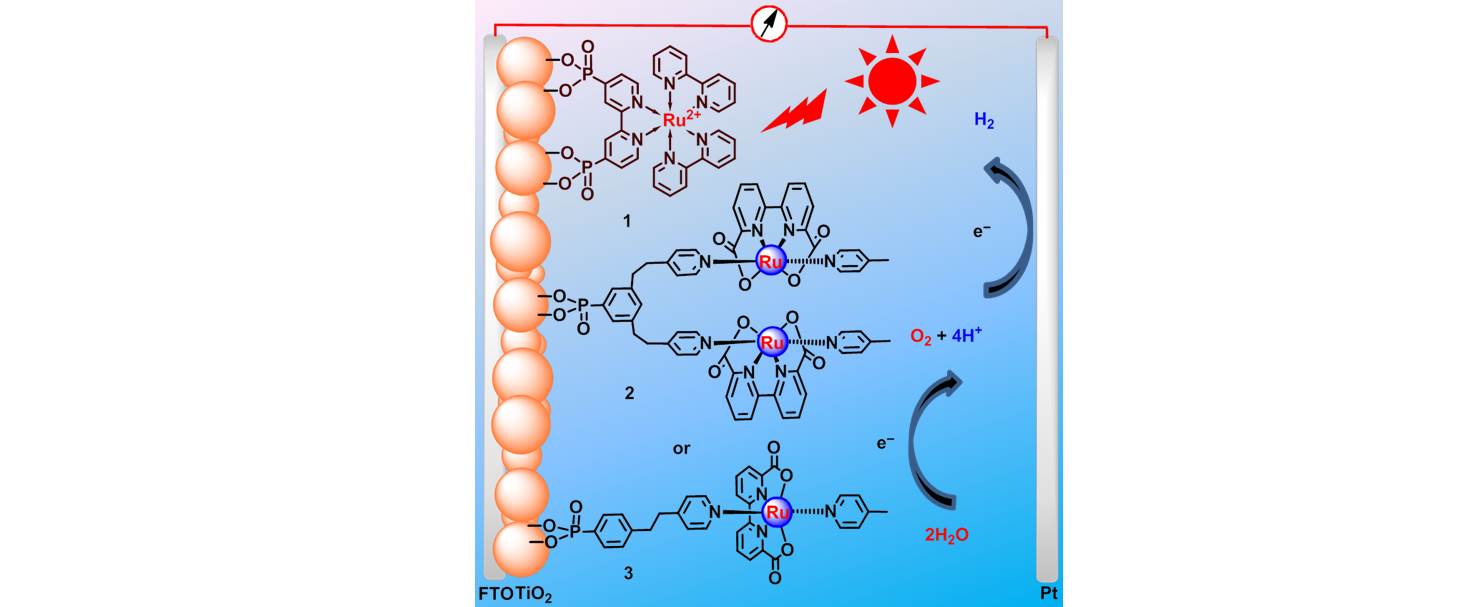
28. Pt-Free tandem molecular photoelectrochemical cells for water splitting driven by visible light
In typical photoelectrochemical (PEC) cells with three-electrode setups, noble metal Pt is often used as the counter electrode to split water into hydrogen and oxygen. However, the high-cost of Pt counter electrode and instability of molecular PEC cells hinder the practical applications. Recently, we have, for the first time, demonstrated a Pt-free tandem molecular PEC cell employing molecular ruthenium- and cobalt- catalysts with strong dipicolinic acid anchoring groups on the respective photoanode and photocathode for the visible light driven total water splitting. The Pt-free tandem molecular PEC cell showed effective and steady photocurrent density of ca. 25 μA cm-2 for water splitting driven by visible light without applying any external bias [Phys. Chem. Chem. Phys. 2014, 16, 25234-25240]. This study indicates that tandem molecular PEC cells can provide great potential to the Pt-free devices for light driven total water splitting.
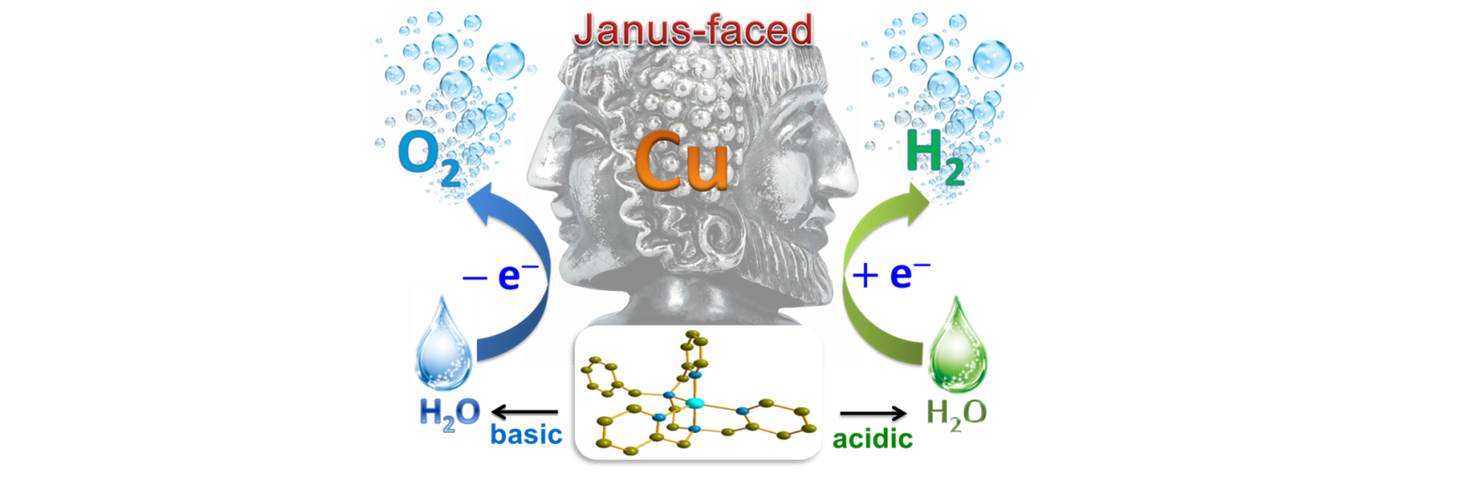
29. High performance photoelectrochemical devices using molecular catalysts for light driven total water splitting
In collaboration with State Key Laboratory of Fine Chemicals, Dalian University of Technology (DUT) through the platform DUT-KTH Joint Education and Research Center on Molecular Devices, we have successfully synthesized a copper complex, [(bztpen)Cu](BF4)2 (bztpen = N-benzyl-N,N',N'-tris(pyridin-2-ylmethyl)ethylenediamine), which revealed to be a Janus-faced molecular catalyst for both electrochemical water oxidation and reduction. It displays high catalytic activity for the electrochemical proton reduction in acidic aqueous solutions, with a calculated hydrogen generation rate constant (kobs) over 10000 s−1. The turnover frequency (TOF), obtained from the CPE experiment of [(bztpen)Cu](BF4)2 in pH 2.5 buffer at –0.90 V over 2 h using a glassy carbon electrode, is 7000 h−1 cm−2 with 96% Faradaic efficiency [P. Zhang et al, Angew. Chem. Int. Ed. 2014, 54, 13803-13807]. For the other face, [(bztpen)Cu]2+ turns out to be a water oxidation catalyst in pH 13 buffer solutions, with an onset overpotential of 350 mV. The anodic current density reaches 3.0 mA cm−2 at +1.2 V vs NHE. The mechanism involving two proton-coupled reduction steps was proposed for the H2 generation reaction catalyzed by [(bztpen)Cu]2+.
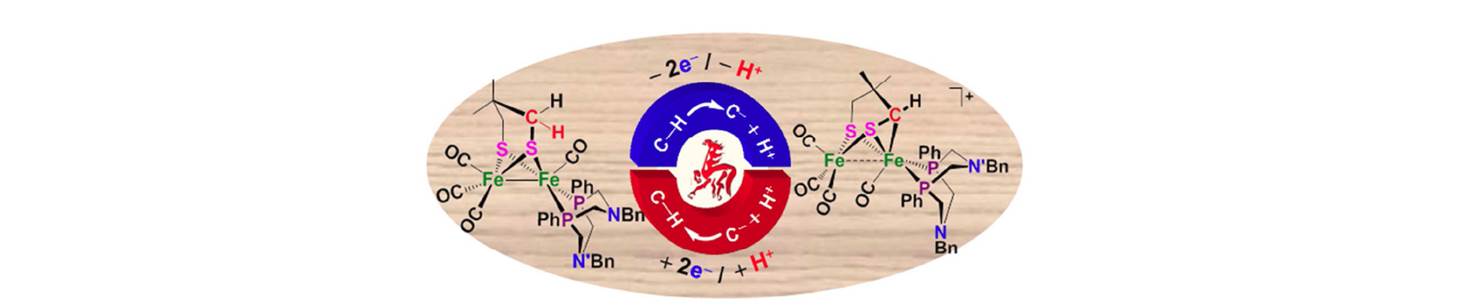
30. Intramolecular iron-mediated C–H bond heterolysis with an assist of internal amine bases in [FeFe]-hydrogenase models
Although many metalloenzymes containing iron play a prominent role in biological C–H activation processes, to date iron-mediated C(sp3)–H heterolysis has not been reported for synthetic models of Fe/S-metalloenzymes. In contrast, ample precedent has established that nature’s design for reversible hydrogen activation by the diiron hydrogenase ([FeFe]-H2ase) active site involves multiple irons, sulfur bridges, a redox switch, and a pendant amine base, in an intricate arrangement to perform H–H heterolytic cleavage. In response to whether this strategy might be extended to C–H activation, we discovered very recently that a [FeFe]-H2ase model demonstrates iron-mediated intramolecular C–H heterolytic cleavage via an agostic C–H interaction, with proton removal by a nearby pendant amine, affording FeII–[(Fe′II–CH–)S] three-membered-ring products, which can be reduced back to 1 by Cp2Co in the presence of HBF4 [D. Zheng et al, J. Am. Chem. Soc. 2014, 136, 16817-16823]. The function of the pendant base as a proton shuttle was confirmed by the crystal structures of the N-protonated intermediate and the final deprotonated product in comparison with that of a similar but pendant-amine-free complex that does not show evidence of C–H activation. The mechanism of the process was backed up by DFT calculations. This work was highlighted by the editor of JACS with a title “Breaking up C and H is now less hard to do” [J. Am. Chem. Soc. 2014, 136, 16698−16699].
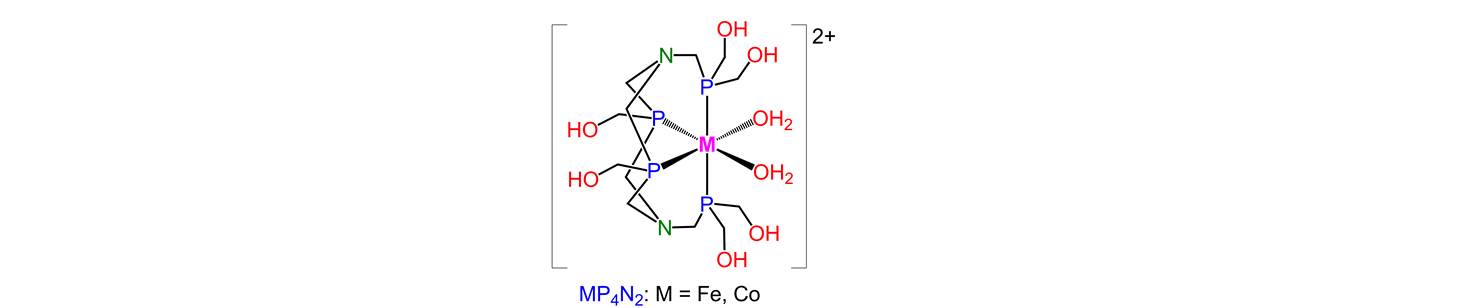
31. A super-efficient cobalt catalyst for electrochemical hydrogen production from neutral water with 80 mV overpotential
To realize the target of storing energy by reduction of water to hydrogen, electrocatalysts that produce hydrogen from neutral water with high activity at low overpotential and with good durability are strongly desired. An inexpensive and easily prepared super-efficient cobalt catalyst was developed in our group, which displays extremely low overpotential (80 mV) while maintaining high activity and good stability for the electrochemical production of hydrogen from neutral water or from sea water [Energy Environ. Sci. 2014, 6, 329-334]. The current density reaches 16 mA cm−2 per geometric surface area at –0.95 V vs. NHE in a phosphate buffer solution of CoP4N2 at pH 7. Bulk electrolysis of CoP4N2 in a neutral aqueous buffer solution at –1.0 V vs. NHE produced 9.24 ´ 104 mol H2 (mol cat)−1 over a period of 20 h with a Faradaic efficiency close to 100%, corresponding to a turnover frequency of 1490 mol H2 (mol cat)−1 h−1 cm−2.
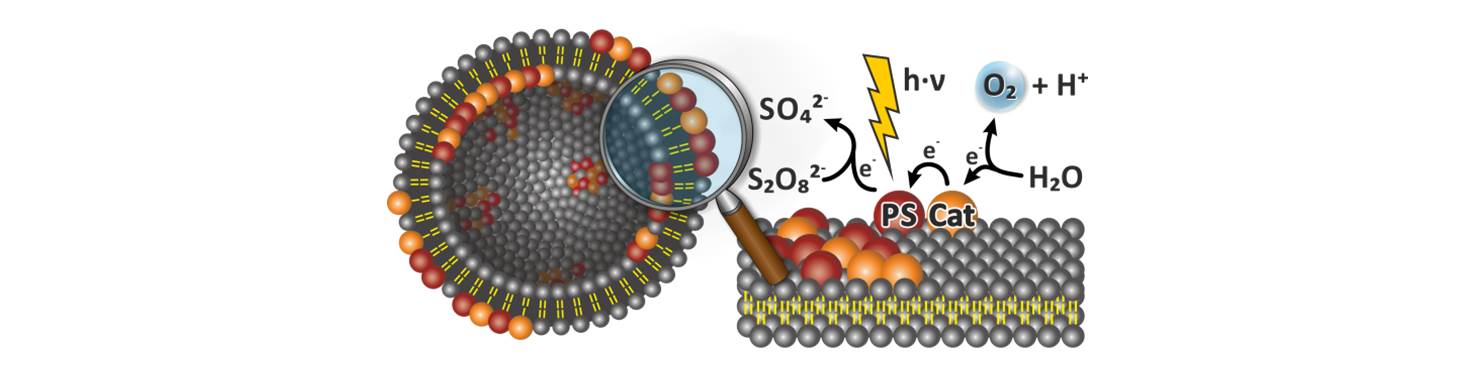
32. Photocatalytic Water Oxidation at Soft Interfaces
Molecular water oxidation catalysts have been, for the first time, co-embedded with a photosensitizer into phospholipid membranes. The functionalized small unilamellar vesicles produce molecular oxygen by photocatalysis when irradiated with visible light in aqueous buffer. The two dimensional assembly of the catalysts at the lipid water interface mimics photoactive membranes in biology and allows a photocatalytic water oxidation at very low catalyst concentrations of 500 nM, which cannot be reached in homogeneous systems. Highest TONs are obtained below the membranes main transition temperature indicating that phase separation, clustering and a limited dynamic enhance the photocatalytic activity of the assembly. The concept of membrane co-embedding can be applied to various combinations, ratios and concentrations of photosensitizers and water oxidizing catalysts, providing a new approach for artificial photosynthesis.
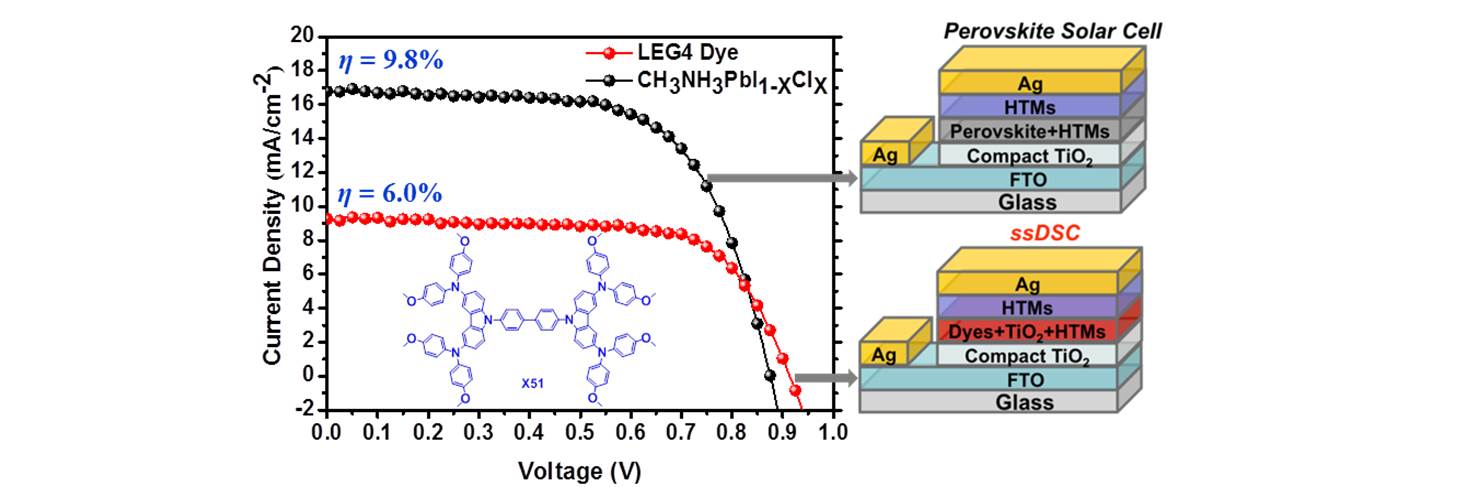
33. Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and perovskite solar cells
Two novel carbazole-based small molecule organic hole-transport materials (HTMs) with straightforward synthetic routes and high hole mobility have been synthesized and investigated for solid-state dye-sensitized solar cells (ssDSCs) and perovskite solar cells (PSCs). The HTM-X51-based devices exhibited higher power conversion efficiency (PCE) of 6.0% than those of the smaller HTM-X19-based devices (4.5%), which also overplayed the PCE of 5.5% obtained by using the state-of-the-art HTM Spiro-OMeTAD [B. Xu et al, Adv. Mater. 2014, 26, 6629-6634]. When making the same comparison in perovskite solar cells, devices based on HTM-X51 exhibited higher power conversion efficiency of 9.8% than those containing HTM-X19 (7.6%); the former results are comparable to the results of 10.2% obtained when using Spiro-OMeTAD. These results qualify the new HTM as promising materials for preparing high-efficiency ssDSCs and PSCs. Moreover, X51 exhibits a higher hole mobility and conductivity than X19, leading to better performance in the device. These results clearly show that molecular engineering is a viable route to develop high hole mobility and high conductivity HTMs for highly efficient ssDSCs and PSCs in the future.
34. Highly efficient bio-inspired molecular Ru water oxidation catalysts with negatively charged backbone ligands
This account describes our endeavors in design of effective Ru WOCs with low overpotential, large turnover number and high turnover frequency by introducing negatively charged ligands, such as carboxylate. Negatively charged ligands stabilized the high valent states of Ru catalysts, as evidenced by the low oxidation potentials. Meanwhile, the oxygen production rates of our Ru catalysts were improved dramatically as well. Thanks to the strongly electron donation ability of carboxylate containing ligands, a seven-coordinate RuIV species was isolated as a reaction intermediate, shielding light on the reaction mechanisms of Ru-catalyzed water oxidation chemistry. Auxiliary ligands have dramatic effects on the water oxidation catalysis in terms of the reactivity and the reaction mechanism. For instance, the Ru-bda (H2bda = 2,2¢-bipyridine-6,6¢-dicarboxylic acid) water oxidation catalysts catalyze CeIV-driven water oxidation extremely fast via the radical coupling of two RuV=O species while the Ru-pda (H2pda = 1,10-phenanthroline-2,9-dicarboxylic acid) water oxidation catalysts catalyze the same reaction slowly via the water nucleophilic attack on a RuV=O species. With numbers of active Ru catalysts in hands, light driven water oxidation were accomplished by using catalysts with low catalytic onset potentials. The structures of molecular catalysts could be readily tailored to introduce additional functional groups, which favors the fabrication of state-of-the-art Ru-based water oxidation devices, such as electrochemical water oxidation anodes and photo electrochemical anodes. The development of efficient water oxidation catalysts has led to a step forward to the sustainable energy system (L. Duan et al, Acc. Chem. Res. 2015, 48, 2084-2096).
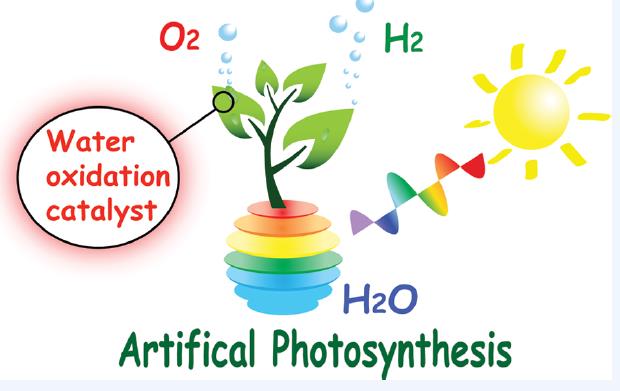
35. Visible Light-Driven Water Oxidation Promoted by Host-Guest Interaction between Photosensitizer and Catalyst with A High Quantum Efficiency
A highly active supramolecular system for visible light-driven water oxidation was developed with cyclodextrin-modified ruthenium complex as the photosensitizer, phenyl-modified ruthenium complexes as the catalysts and sodium persulfate as the sacrificial electron acceptor. The catalysts were found to form 1:1 host-guest adducts with the photosensitizer. Stopped-flow measurement revealed the host-guest interaction is essential to facilitate the electron transfer from catalyst to sensitizer. As a result, a remarkable quantum efficiency of 84% was determined under visible light irradiation in neutral aqueous phosphate buffer. This value is nearly one order of magnitude higher than that of non-interaction system, indicating that the non-covalent incorporation of sensitizer and catalyst is an appealing approach for efficient conversion of solar energy into fuels (H. Li et al, J. Am. Chem. Soc. 2015, 137, 4332-4335).
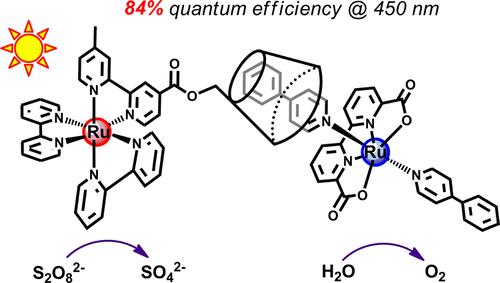
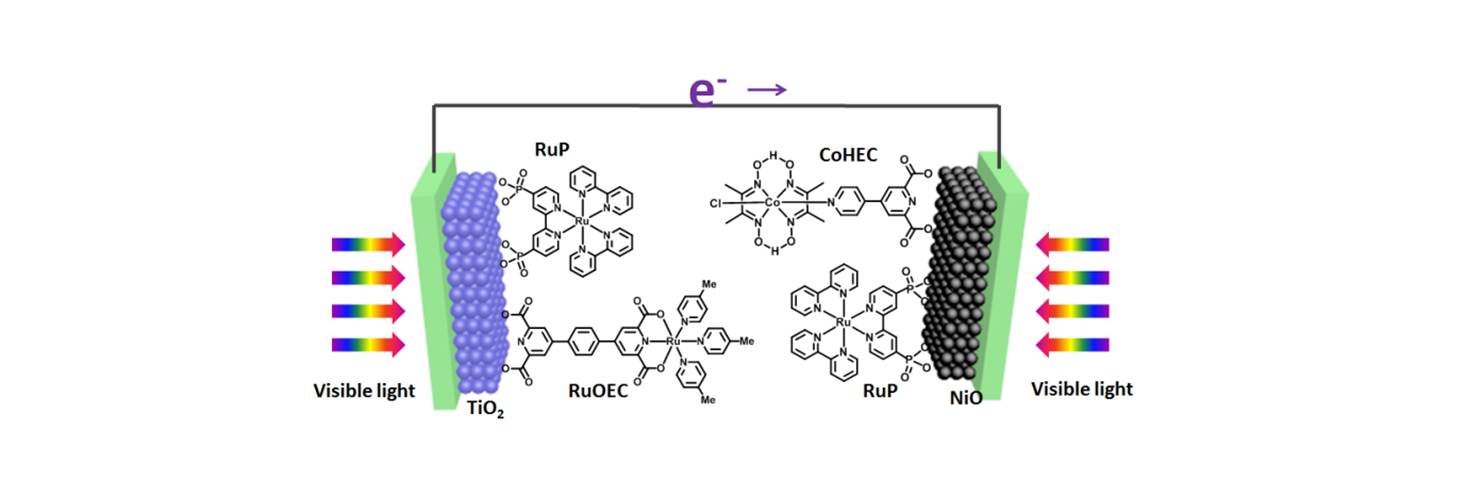
36. An Organic dye-sensitized tandem photoelectrochemical cell for light driven water splitting
Light driven water splitting was achieved by a tandem dye-sensitized photoelectrochemical cell with two photo-active electrodes. The photoanode is constituted by an organic dye L0 as photosensitizer and a molecular complex Ru1 as water oxidation catalyst on meso-porous TiO2, while the photocathode is constructed with an organic dye P1 as photo-absorber and a molecular complex Co1 as hydrogen generation catalyst on nanostructured NiO. By combining the photocathode and the photoanode, this tandem DS-PEC cell can split water by visible light under neutral pH conditions without applying any bias (F. Li et al, J. Am. Chem. Soc. 2015, 137, 9153-9159).
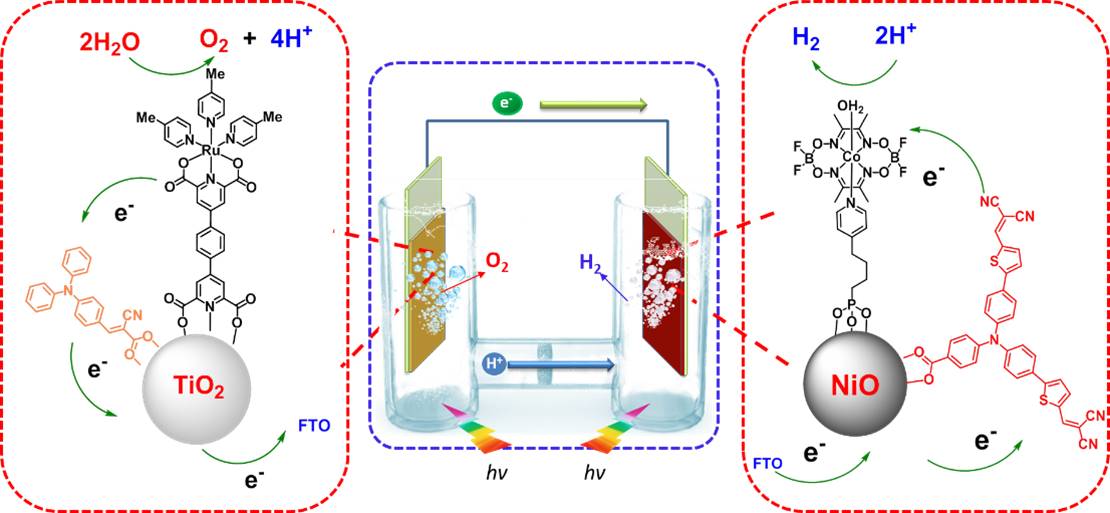
37. Electroless plated Ni-Bx films as highly active electrocatalysts for hydrogen production from water over a wide pH range
The performance of electroless plated Ni–Bx films was studied in a wide pH range for the hydrogen evolution reaction(HER). The atomic ratio of B to Ni has great influence on the particle size and the morphology of Ni–Bx materials, and more importantly on the catalytic H2- evolution property of Ni–Bx films. The film with a B:Ni atomic ratio of 0.54, denoted as Ni–B0.54, displayed the best performance with a current density of 10 mAcm-2 at very low overpotentials (η) of 45 mV in 0.5 M H2SO4, 54 mV in 1.0 M pH7 phosphate buffer solution (PBS),and 135 mV in 1.0 M KOH,and the catalytic activity maintained over 20-h electrolysis at η=100 mV in all tested media of different pH values. The Tafel slopes of the Ni–B0.54 film are 43, 77, and 88 mVdec-1 in 0.5 M H2SO4, 1.0 M neutral PBS, and 1.0 M KOH, respectively. These results show that the combination of earth-abundant nickel and boron elements in an optimal B-to-Ni atomic ratio can provide highly active and stable electrocatalysts for the HER over a wide pH range (P. Zhang et al, Nano Energy. 2016, 19, 98-107).
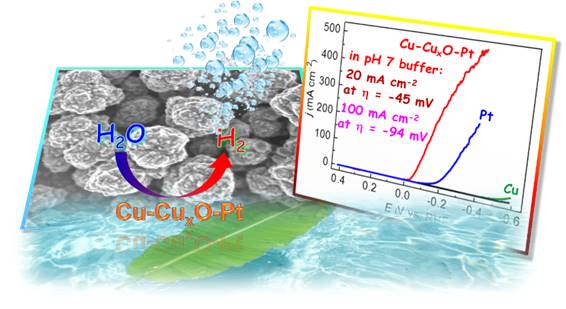
38. Super-Active and Robust Platinum-Doped Copper Nanoparticulate Films for Electrochemical H2 Production from Neutral Water
Electrocatalysts that are stable and highly active at low overpotential (η) under mild conditions as well as cost-effective and scalable are eagerly desired for potential use in photo- and electro-driven hydrogen evolution devices. Here the fabrication and characterization of a super-active and robust Cu-CuxO-Pt nanoparticulate electrocatalyst is reported, which displays a small Tafel slope (44 mV dec−1) and a large exchange current density (1.601 mA cm−2) in neutral buffer solution. The catalytic current density of this catalyst film reaches 500 mA cm−2 at η = −390 ± 12 mV and 20 mA cm−2 at η = −45 ± 3 mV, which are significantly higher than the values displayed by Pt foil and Pt/C electrodes in neutral buffer solution and even comparable with the activity of Pt electrode in 0.5 m H2SO4 solution (P. Zhang et al, Adv. Energy Mater. 2016, 6, 1502319).
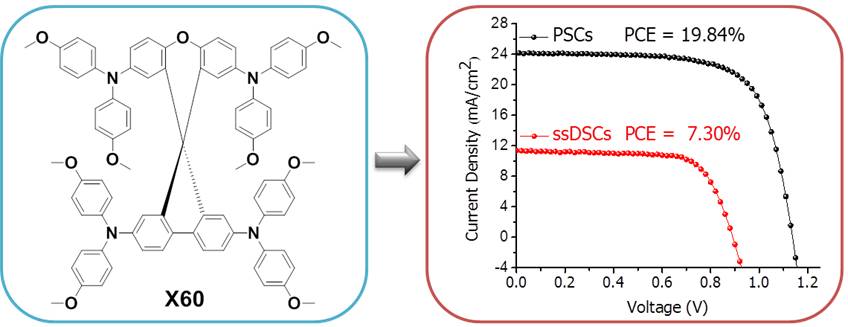
39. Facile Synthesis of A Spiro[fluorene-9,9′-xanthene]-based Hole Transport Material for Efficient Solid-state Dye-sensitized Solar Cells and PerovskiteSolar Cells
A low-cost spiro-[fluorene-9,9′-xanthene] (SFX) based organic hole transport material (HTM) termed X60 with a two-step synthetic route was designed and synthesized. Devices with X60 as HTM showed high power conversion efficiencies (PCEs) amounting to 7.30% in solid-state dye-sensitized solar cells (ssDSCs) and to 19.84% in perovskite solar cells (PSCs), respectively, under 100 mW∙cm-2 AM1.5G solar illumination. To the best of our knowledge, this is the first example of an easily synthesized spiro structured HTM showing comparable performance with respect to the well-known HTM-Spiro-OMeTAD in both ssDSCs and PSCs. Furthermore, the facile synthesis of X60 with commercially available starting materials makes this HTM very promising for large-scale industrial production in the future (B. Xu et al, Energy Environ. Sci. 2016, 9, 837-877).
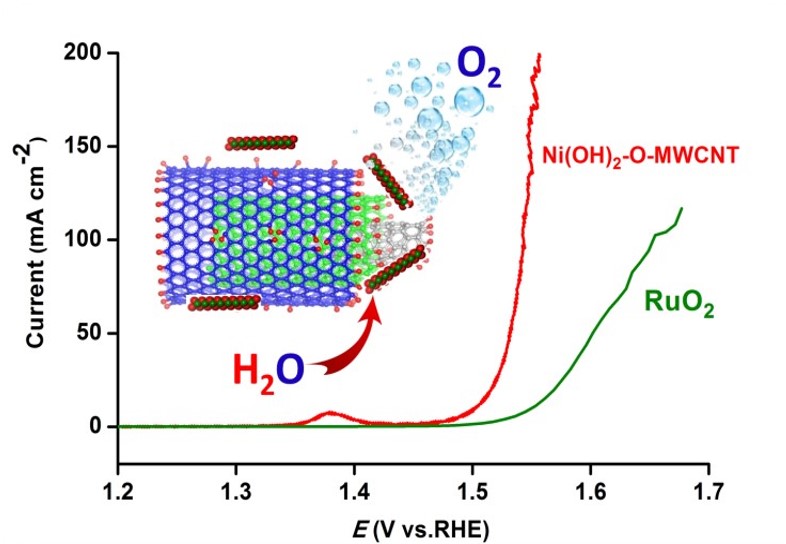
40. Promoting the Water Oxidation Catalysis by Synergistic Interactions between Ni(OH)2 and Carbon Nanotube
Aiming for highly efficient and cost-effective electrocatalysts based on non-precious material for solar fuel production, we have developed a new hybrid oxygen evolution electrocatalyst by densely embedding β−Ni(OH)2 nanoplates into mildly oxidized and hydrothermal treated multiwall carbon nanotubes. Attributed to strong synergistic effect, this hybrid catalyst efficiently catalyzes oxygen evolution reactions (OER) with an overpotential of only 270 mV vs. RHE to achieve a current density of 10 mA cm–2 in 1 M KOH solution. To our surprise, a very small Tafel slope of 32 mV dec–1 is obtained for this catalyst. Moreover, this hybrid catalyst exhibits high Faraday efficiency (~96%) and excellent stability during bulk electrolysis (L. Wang et al, Adv. Energy Mater. 2016, 6, 1600516).
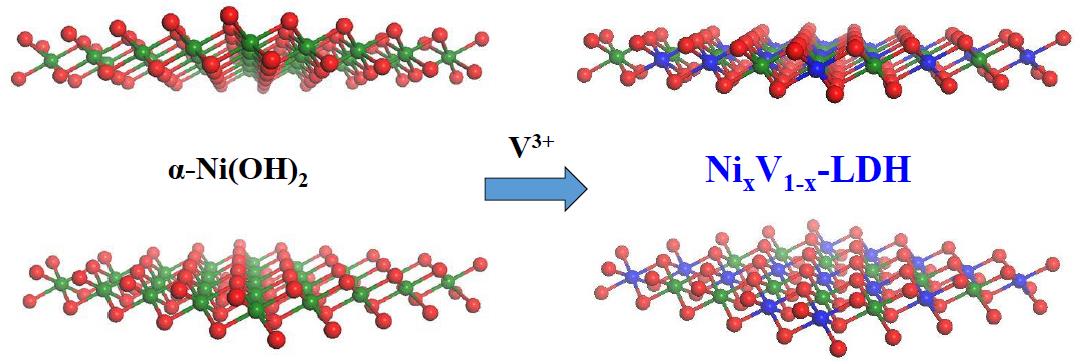
41. Nickel-Vanadium Monolayer Double Hydroxide for Efficient Electrochemical Water Oxidation
Highly active and low-cost electrocatalysts for water oxidation are urgently desired due to the demands on sustainable solar fuels, while developing highly efficient catalyst to meet the industrial requirement is still challenging. Herein, we report a monolayer of nickel-vanadium layered double hydroxide which shows a current density of 27 mA cm-2 (57 mA cm-2 after ohmic-drop correction) at an overpotential of 350 mV for water oxidation. Such performance is comparable to those of the best-performing nickel-iron layered double hydroxides for water oxidation in alkaline media. Mechanism study indicates that the nickel-vanadium LDHs can lead to high intrinsic catalytic activity, mainly due to the good conductivity, facile electron transfer and sufficient active sites. This work exhibits a very promising vanadium involved layered double hydroxide with highly catalytic activity, and expands the scope of cost-effective electrocatalysts for water splitting (K. Fan et al, Nature Commun. 2016, 7, 11981).
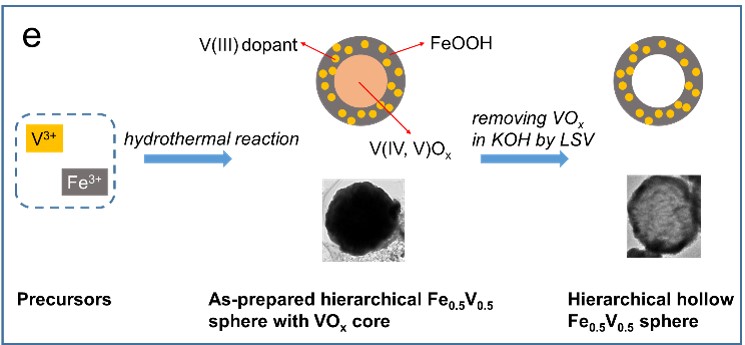
42. Hollow Iron-Vanadium Composite Spheres: A Highly Efficient Fe-Based Water Oxidation Electrocatalyst without Need of Ni or Co
Noble metal-free bimetal-based electrocatalysts have shown high efficiency for water oxidation. Ni and/or Co in these electrocatalysts are essential to provide a conductive, high-surface area and chemically stable host. However, the necessity of Ni or Co limits the scope of low-cost electrocatalysts. Herein, we report a hierarchical hollow FeV composite, which is Ni and Co-free and highly efficient for electrocatalytic water oxidation with low overpotential 390 mV (10 mA cm-2 catalytic current density), low Tafel slope of 36.7 mV dec-1 and a considerable durability. This work provides a novel and efficient catalyst, and greatly expands the scope of low-cost Fe-based electrocatalysts for water splitting without need of Ni or Co (K. Fan et al, Angew. Chem. Int. Ed. 2017, 56, 3289-3293).
43. Re-investigation of Cobalt porphyrin for Electrochemical Water Oxidation on FTO Surface: Formation of CoOx as Active Species
The use of cobalt porphyrin complexes as efficient and cost effective molecular catalysts for water oxidation has previously been investigated. However, by combining a set of analytical techniques (electrochemistry, Ultraviolet–visible spectroscopy (UV-Vis), Scanning Electron Microscopy (SEM), Energy-dispersive X-ray spectroscopy (EDS) and Synchrotron based Photoelectron spectroscopy (SOXPES and HAXPES), we have demonstrated that under certain conditions (borate buffer pH 9.2) the aforementioned catalysts, deposited on FTO glasses, decomposed promptly into a thin film of CoOx, presumably CoO, on the surface of the electrode, which can only be detected by SOXPES, while conventional techniques are ineffective. This newly formed film represents a high turnover frequency (TOF), whilst the high transparency of CoOx based electrode is very promising for future application in photoelectrochemical cells (Q. Daniel et al, ACS Catal. 2017, 7, 1143-1149).
44. Highly Efficient Photoelectrochemical Water Oxidation with Immobilized Molecular Co4O4 Cubane Catalyst on BiVO4
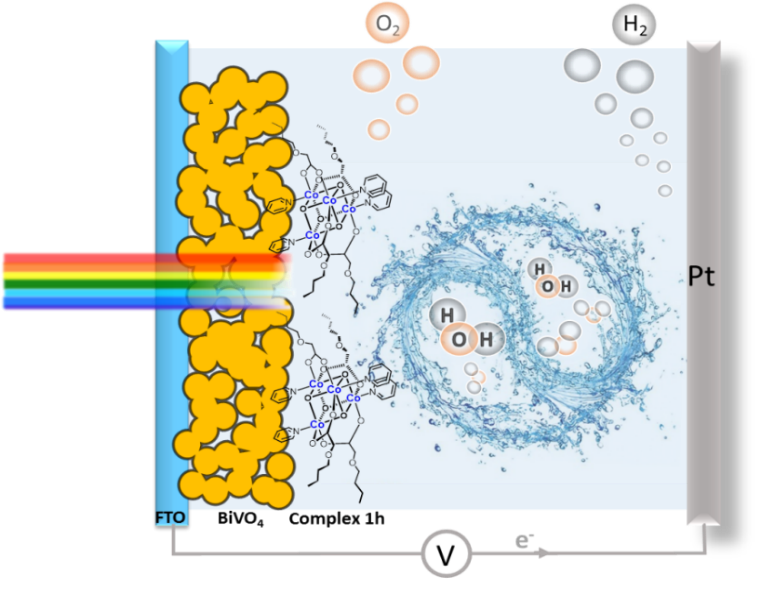
Molecular Co4O4 cubane water oxidation catalysts were combined with BiVO4 electrodes for photoelectrochemical (PEC) water splitting. The results show that tuning the substituent groups on cobalt cubane allows the PEC properties of the final molecular catalyst/BiVO4 hybrid photoanodes to betailored. Upon loading a new cubane complex featuring alkoxy carboxylato bridging ligands (1 h) on BiVO4, an AM 1.5 G photocurrent density of 5 mA/cm2 at 1.23 V vs. RHE for water oxidation was obtained, the highest photocurrent for undoped BiVO4 photoanodes. A high solar-energy conversion efficiency of 1.84% was obtained for the integrated photoanode, a sixfold enhancement over that of unmodified BiVO4. These results and the high surface charge separation efficiency support the role of surface-modified molecular catalysts in improving PEC performance and demonstrate the potential of molecule/semiconductor hybrids for efficient artificial photosynthesis (Y. Wang et al, Angew. Chem. Int. Ed. 2017, 56, 6911-6915).
45. Efficient Perovskite Solar Cells Based on a Solution Processable Nickel(II) Phthalocyanine and Vanadium Oxide Integrated Hole Transport Layer
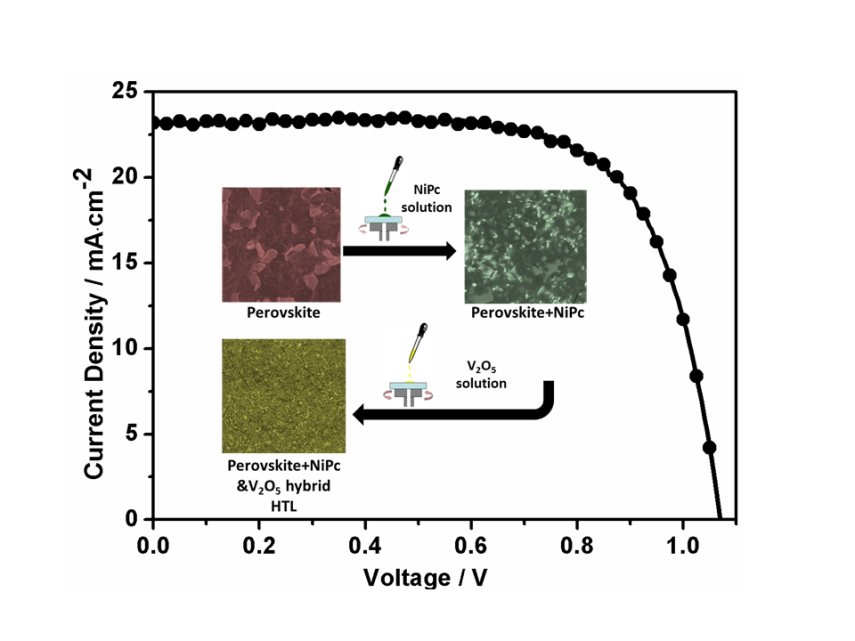
An organic-inorganic integrated hole transport layer (HTL) composed of the solution processable nickel phthalocyanine (NiPc) abbreviated NiPc-(OBu)8 and vanadium(V) oxide (V2O5) is successfully incorporated into structured mesoporous perovskite solar cells (PSCs). The optimized PSCs show the highest stabilized power conversion efficiency (PCE) of up to 16.8% and good stability under dark ambient conditions. These results highlight the potential application of organic-inorganic integrated HTLs in PSCs (M. Cheng et al, Adv. Energy Mater. 2017, 7, 1602556).
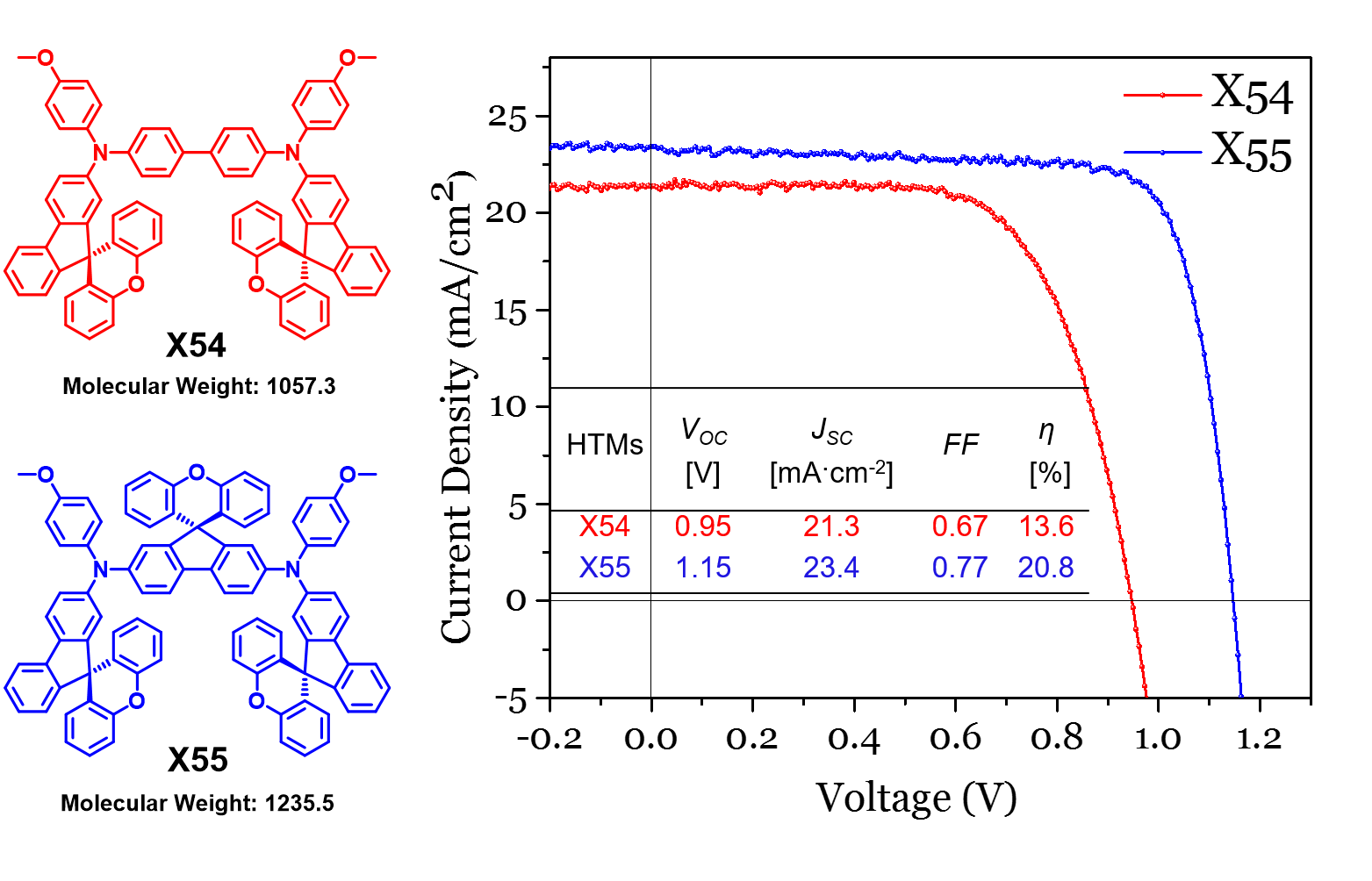
46. Tailor-Making Low-Cost Spiro[fluorene-9,9'-xanthene]-Based 3D Oligomers for Perovskite Solar Cells
Two spiro[fluorene-9,9’-xanthene] (SFX)-based 3D oligomers termed X54 and X55 were tailor-made by using a one-pot synthesis approach and applied them in PSCs. One of the HTMs, X55, constructed of three SFX units, shows an excellent 3D structure and much better film forming ability as compared to those of Spiro-OMeTAD and X54. PSC devices based on X55 as HTM show a very impressive PCE of 20.8% under 100 mW∙cm-2 AM1.5G solar illumination, which is much higher than the PCE of the reference devices based on Spiro-OMeTAD (18.8%) and X54 (13.6%) under the same conditions (B. Xu et al, Chem. 2017, 2, 676-687).
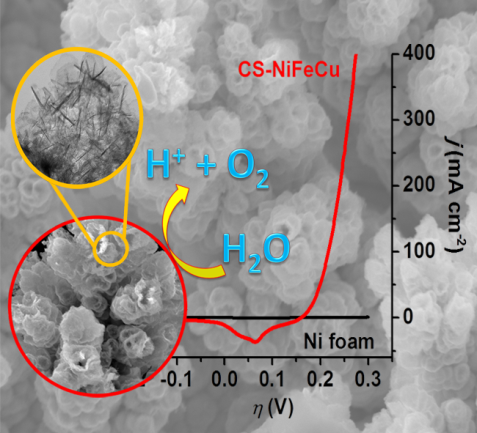
47. Dendritic Core-Shell NiFeCu Metal/Metal Oxide Electrode for Efficient Electrocatalytic Water Oxidation
Electrochemical water splitting requires efficient water oxidation catalysts to accelerate the sluggish kinetics of water oxidation reaction. Here we report a promisingly dendritic core-shell NiFeCu metal/metal oxide (CS-NiFeCu) electrode, prepared via dealloying with an electrodeposited NiFeCu alloy as a precursor, as the catalyst for water oxidation. The as-prepared CS-NiFeCu electrode was characterized with porous oxide shells and metallic cores. The porous oxide shell with its high electrochemically active surface area is responsible for the catalytic activity, while the metallic cores work as facile electron transport highways. This tri-metal based CS-NiFeCu electrode exhibits a remarkable activity towards water oxidation in alkaline medium with an overpotential of only 180 mV at a current density of 10 mA cm-2. The CS-NiFeCu electrode exhibits pH-dependent oxygen evolution reaction (OER) activity on the reversible hydrogen electrode scale, suggesting that non-concerted proton-electron transfers participate in catalyzing the OER. To the best of our knowledge, the as-fabricated CS-NiFeCu is one of most promising oxygen evolution catalysts. The parent alloy based synthetic approach described here is applicable to the synthesis of other metal and/or metal oxides-based nanomaterials (P. Zhang et al, Nature Commun. 2018, 9, in press).
48. Device Fabrication for Water Oxidation, Hydrogen Generation and CO2 Reduction via Molecular Engineering
Research on the storage of solar energy in terms of hydrogen or carbon-based fuels by using sunlight to split water or to reduce CO2, respectively, has gained significant attention in recent years. Among reported water splitting systems, one approach has focused on hybrid systems with molecular catalysts or molecular light-harvesting systems that are combined with nanostructured materials. In this review we summarize recent developments in operation and fabrication strategies for various water splitting devices constructed from electrodes (electrochemical cells) or photoelectrodes (photoelectrochemical cells) using molecular engineering. We also provide insights into the factors that influence device’s efficiency and stability, and provide guidelines for future fabrication strategies for more advanced devices (F. Li et al, Joule 2018, 2, 36-60).