Bio and Life Science
Large-scale research infrastructures such as synchrotrons, free electron lasers (FELs), and neutron and muon sources play a pivotal role in advancing bio- and life-sciences by enabling scientists to explore biological systems at unprecedented spatial and temporal resolutions.
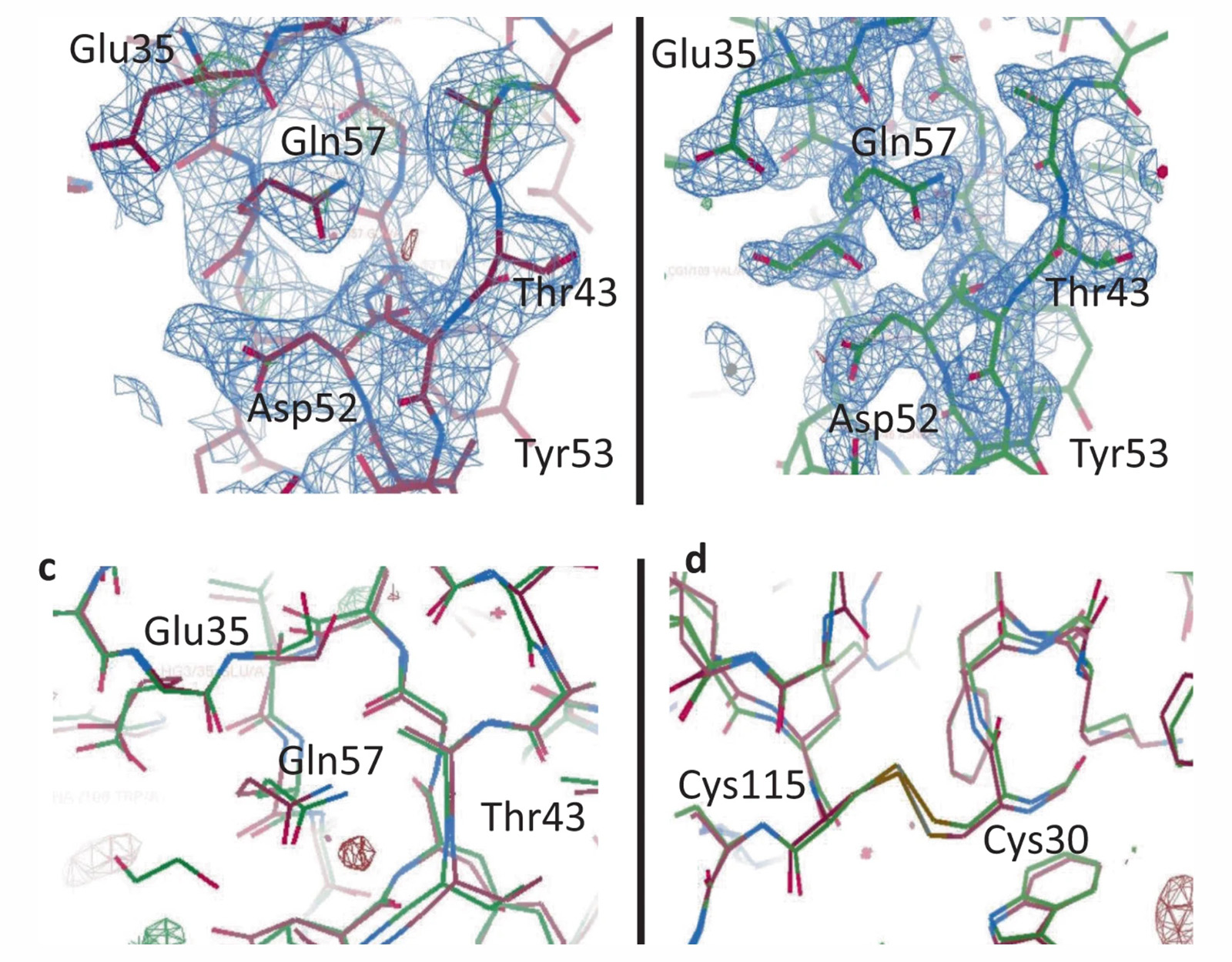
Large-scale research infrastructures make it possible to visualize the three-dimensional structures of biomolecules at atomic resolution, providing fundamental insights into their function, interactions, and dynamics — insights that are essential for understanding disease mechanisms and developing new therapeutic strategies. Synchrotron radiation and FELs, for example, produce highly intense and tunable X-ray beams that allow for detailed structural studies of biomolecules such as proteins, nucleic acids, and complex macromolecular assemblies.
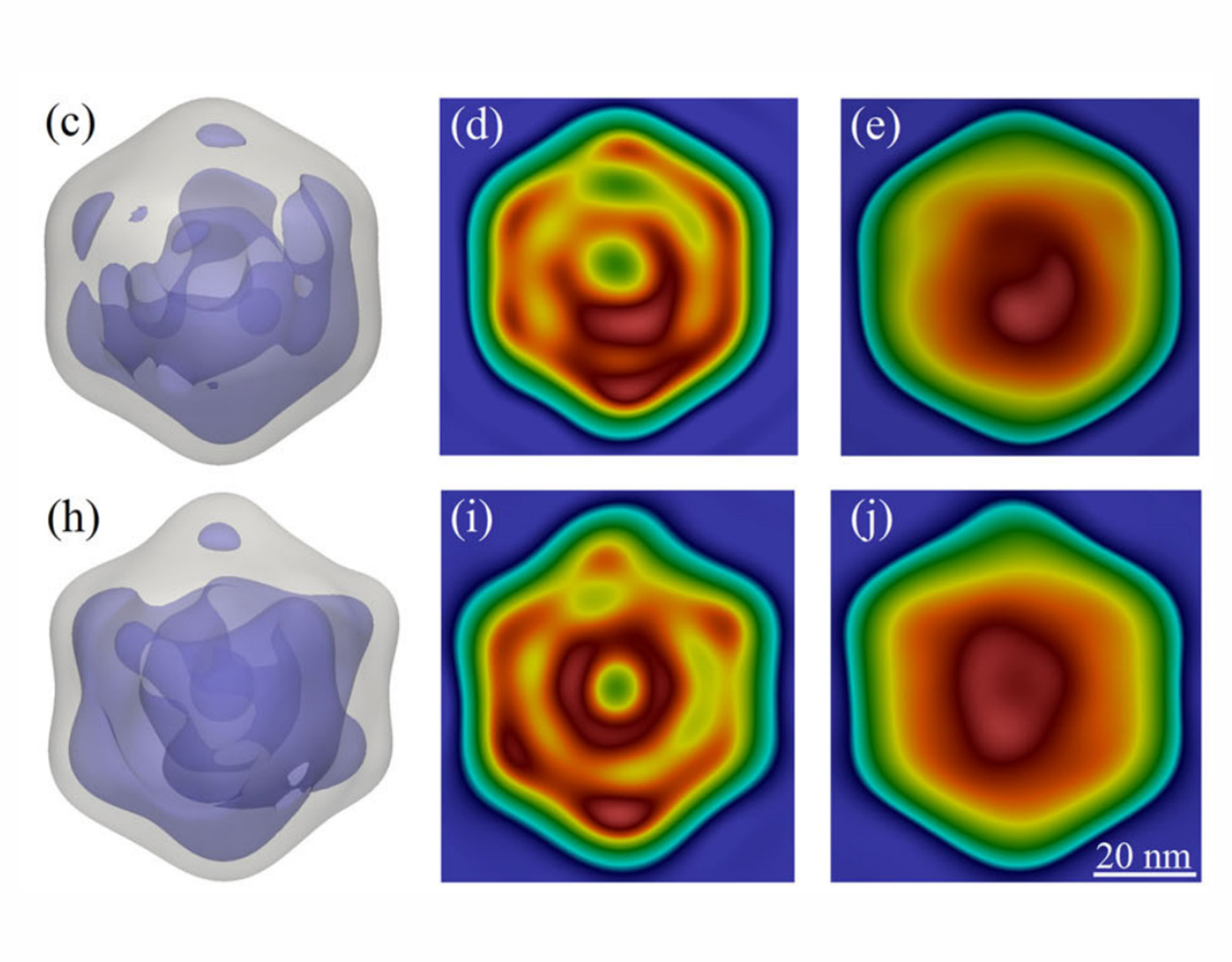
Neutron and muon sources complement these X-ray-based techniques by offering unique sensitivity to light elements, such as hydrogen, which are crucial in biological systems but often difficult to detect with X-rays. Neutrons, in particular, are ideal for studying hydration, lipid membranes, and large biological complexes in their near-native states, helping to elucidate how structure and function are maintained under physiological conditions. Meanwhile, muon-based techniques can probe redox processes and transient magnetic properties in biochemical reactions, expanding our understanding of enzymatic mechanisms and metabolic pathways.
Together, these large-scale facilities provide a powerful and synergistic suite of tools that drive innovation in structural biology, molecular medicine, drug discovery, and biotechnology. They also foster interdisciplinary collaboration by bringing together biologists, chemists, physicists, and computational scientists to address complex questions about life at the molecular and cellular levels. As such, synchrotrons, FELs, and neutron/muon sources form the backbone of modern biological research infrastructures, transforming the way we visualize and manipulate the molecular machinery of life.
The European Spallation Source (ESS) and MAX IV Laboratory together represent a major leap forward for Sweden’s capacity in life-science research. MAX IV, already one of the most advanced synchrotron radiation facilities in the world, offers exceptionally bright and coherent X-rays that enable imaging and spectroscopy techniques far beyond previous generations. This makes it possible to study biomolecular structures, tissues, and cells in real time and at near-atomic resolution. The ESS, once fully operational, will be the world’s most powerful neutron source, providing unmatched opportunities to study biological systems in conditions close to their natural environments — for instance, probing hydrogen bonding, membrane dynamics, and protein folding. The combination of these two infrastructures within close proximity in Lund establishes Sweden as a global hub for cutting-edge life-science research. Together, they will not only attract international collaborations and talent but also drive innovation across medicine, pharmacology, and biotechnology, strengthening Sweden’s position as a leader in molecular and structural biology.
Examples