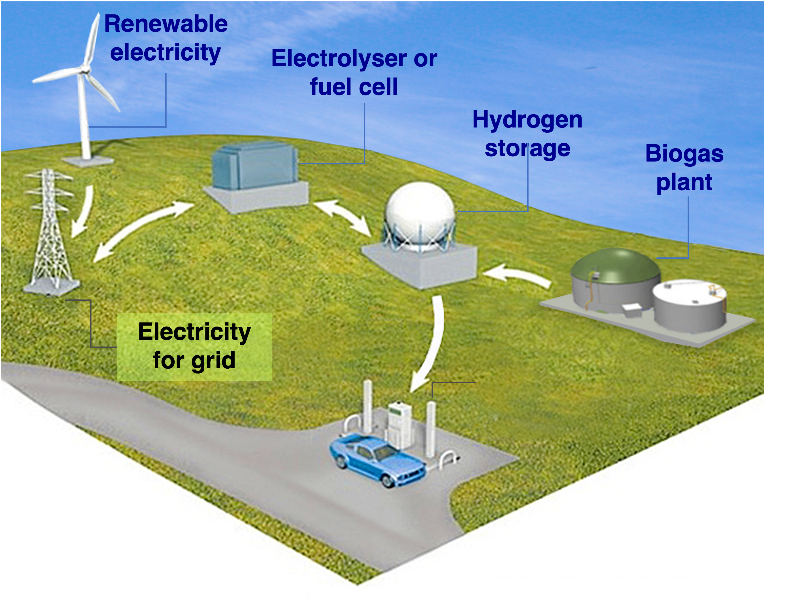
The course mainly addresses following areas:
- Production of hydrogen: functional principles, materials, design, properties and performance of different types of electrolysers for hydrogen production and comparisons with other hydrogen production methods.
- Storage and distribution of hydrogen: comparison of different technical solutions.
- Use of hydrogen gas: functional principles, materials, design, properties and performance of different types of fuel cells. Use of hydrogen for transport, industry, the electricity grid and the production of fuels and chemicals.
- The hydrogen society: system integration, socio-economic and political aspects, safety, circularity and sustainability.