The course focuses on equilibrium calculations of acid / base, gas, solubility, complex and redox reactions and practical wet chemical laboratory work in inorganic reaction theory. The course aims to give a general understanding of how the driving forces in nature, i.e. how the strive towards equilibrium, give rise to chemical reactions and how we can practically utilize this concept to influence a reaction.
KD1510 Chemical Equilibrium 6.0 credits
This course will be discontinued.
Last planned examination: Spring 2027
Decision to discontinue this course:
No information inserted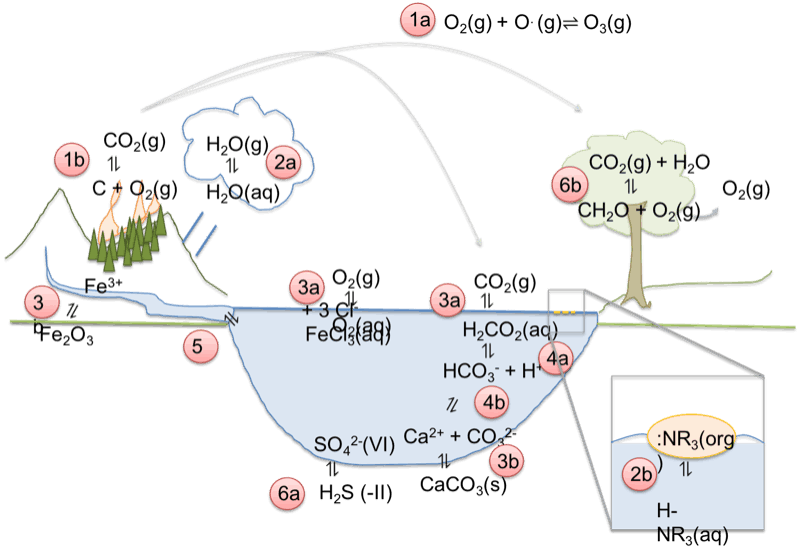
Information per course offering
Course offerings are missing for current or upcoming semesters.
Course syllabus as PDF
Please note: all information from the Course syllabus is available on this page in an accessible format.
Course syllabus KD1510 (Spring 2025–)Content and learning outcomes
Course contents
Intended learning outcomes
On completion of the course, the student should be able to:
- Calculate different types of chemical equilibrium equations with appropriate approximations and predict how the equilibrium concept can be utilized to influence a process (TEN1).
- Independently plan and perform wet chemical work in accordance with standard procedures for safe handling of chemicals and apply the equilibrium concept for analysis and separation (LAB1).
- Identify natural forms of our most common elements in soil, water and air, and based on the principles of equilibrium describe flows and circles in nature (TEN1).
Literature and preparations
Specific prerequisites
Completion of upper-secondary school by 1 July 2011 and adult education at the upper-secondary level (gymnasium) by 1 July 2012 Specific entry requirements: mathematics E, physics B and chemistry A. Passed or 3 in each of the subjects is required.
Upper-secondary school from 1 July 2011 and adult education at upper-secondary level (gymnasium) from 1 July 2012 (Gy2011) Specific entry requirements: Physics 2, Chemistry 1 and Mathematics 4. A pass in each of the subjects is the lowest acceptable grade.
Literature
Examination and completion
Grading scale
Examination
- TEN1 - Examination, 3.0 credits, grading scale: A, B, C, D, E, FX, F
- LAB1 - Laboratory Exercises, 3.0 credits, grading scale: P, F
Based on recommendation from KTH’s coordinator for disabilities, the examiner will decide how to adapt an examination for students with documented disability.
The examiner may apply another examination format when re-examining individual students.
If the course is discontinued, students may request to be examined during the following two academic years.
Other requirements for final grade
All parts in the course should be approved
Examiner
Ethical approach
- All members of a group are responsible for the group's work.
- In any assessment, every student shall honestly disclose any help received and sources used.
- In an oral assessment, every student shall be able to present and answer questions about the entire assignment and solution.