Kursens mål är att ge en introduktion till organisk kemi vad gäller struktur och reaktivitet, praktiskt syntesarbete samt grön kemi. Kursen ger också en stark bas för vidare fördjupning inom organisk kemi.
Kort beskrivning av innehåll:
- Nomenklatur
- Konformation/konfiguration
- Reaktionsmekanismer
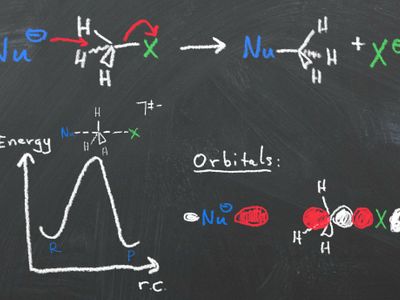
- Frontorbitalteori
- Protontransfer/pKa
- Substitution/elimination
- Additionsreaktioner
- Grön kemi
Detaljerad beskrivning av innehåll:
- det ”organisk-kemiska språket” dvs. återge organiska strukturer grafiskt, namnge organiska föreningar enligt IUPAC-nomenklaturen, trivialnamnen för vissa vanligt förekommande föreningar samt beskriva organiska föreningars tredimensionella struktur grafiskt och enligt CIP-nomenklaturen.
- identifiering och rangordning av nukleofiler, elektrofiler, syror och baser i en kemisk reaktion.
- syra/basjämvikter / pKa / protontransfer.
- mekanismpilens innebörd samt använda den för att beskriva reaktionsmekanismer.
- frontorbitalbegreppet för att kategorisera vilka orbitaler som är HOMO respektive LUMO i organiska molekyler samt att kunna använda dessa resonemang för att avgöra utgången av en reaktion.
- hur reaktionsbetingelser påverkar om en reaktion följer en SN1, SN2, E1 eller E2 mekanism samt det stereokemiska och regiokemiska resultatet. Omvänt ska även reaktionsbetingelser kunna kombineras för att styra en reaktion via en SN1, SN2, E1 eller E2 mekanism.
- organiska föreningars stereokemi och därifrån dra slutsatser om dess konformation och reaktivitet.
- reaktionsmekanismen och det stereokemiska och regiokemiska utfallet vid addition av elektrofiler till alkener.
- begreppet grön kemi och dess applicering i organisk kemi och hur detta kan verka för hållbar utveckling.
- grundläggande spektroskopiska analysmetoder (NMR) för strukturanalys av organiska föreningar.
- risk- och säkerhetsanalys av en organisk-kemisk reaktionsprocess samt kunna och förstå de säkerhetsföreskrifter som krävs vid laborativt arbete.
- omsättning av recept till en genomförd syntes, vilket inkluderar; sätta upp en reaktion, arbeta upp en reaktionsblandning samt rena en förening med hjälp av extraktion, destillation och kristallisation.
- strukturbestämning och karakterisering organiska föreningar med hjälp av vanliga analysmetoder (smältpunkt, NMR, IR).