Targeting angiogenesis using artificial ligands
Angiogenesis is essential in several pathological conditions, including tumor growth and metastasis and also in different ocular disorders. Major regulators include members of the vascular endothelial growth factor (VEGF) family and their receptors (VEGFRs). Targeting the vasculature using protein-based therapeutics primarily aims to normalize the chaotic and/or increased vessel formation by inhibiting dysfunctional pathways. Directly targeting the receptor instead of one of the ligands has the potential advantage of preventing activation by all ligands. For ophthalmic diseases, a challenge is the delivery of protein therapeutics to the eye, which with the current antibody-based therapeutics requires administration by intraocular injection. Smaller antagonistic agents might thus be preferable, potentially allowing for alternative routes of administration, such as topical delivery using eye drops. Moreover, in fields like regenerative medicine, tissue engineering and stem cell research, specific control over the activation of receptors (e.g. VEGFR2) is important and natural ligands are often suboptimal due to their cross-reactive binding. Hence, highly specific artificial ligands for agonistic activation of VEGFR2 should be valuable in such areas.
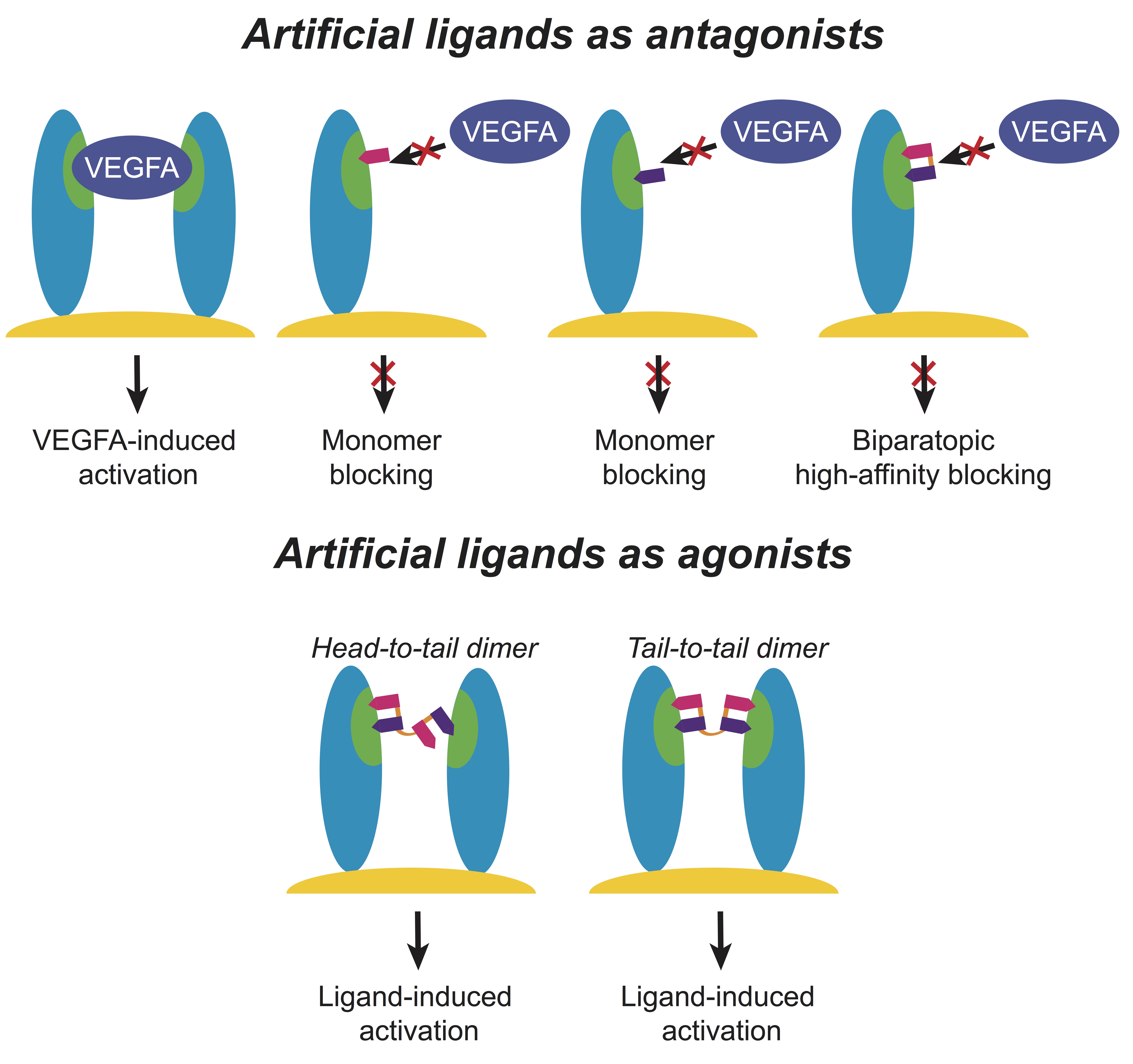
In the project, we are engineering and exploring so-called “biparatopic” artificial ligands for VEGFR2 with an unusual mechanism of binding. The new ligands recognize two distinct adjacent interaction sites on the receptor, analogous to a paper staple. Affinity is key for blocking protein-protein interactions and the new biparatopic format is an efficient strategy to achieve the required affinity. By fusing two separately isolated affinity molecules into one construct, we gained an over 1000-fold improvement in affinity (low pM). They show no crossreactivity for VEGFR1 and 3 and block VEGFs from interacting with VEGFR2. Blocking inhibits ligand-induced phosphorylation and vessel formation in in vitro assays. We anticipate that its performance in several aspects will be superior to that of a regular antibody, particularly with regard to: biodistribution, dose response, tissue penetration, clearance in molecular imaging and administration routes. In addition, recent results show that genetic fusions of two ligands in a homodimeric format shifts the response from antagonistic to agonistic, thereby potentially suitable for specific activation of VEGFR2 signaling.
Research funded by: The Swedish Cancer Society