My Hedhammar
Professor
Details
Researcher
About me
Motivation for our research
Still, a lot of things are unknown about how a disease such as cancer is developing and how it can be cured. We construct physiologically relevant models of various tissue by letting proteins self-assemble into a matrix providing an in vivo-like environment for cells to grow into functional tissue constructs in the lab. These tissue models can be used to better understand disease mechanisms, facilitate drug development, and allow personalized medicine.
Silk proteins as flexible building blocks
Silk proteins have a unique propensity to assemble their chains in an ingenious way so that a strong, elastic, and biocompatible material is formed. We have surveyed the silk assembly mechanism, and are developing methods to control it to obtain desired formats, e.g. fibers, fibrillar networks, and membranes (Fig. 1).
With the use of biotechnology methods, it is feasible to combine various functional molecules. We have developed methods to functionalize silk either at the genetic or protein level. This allows us to design and construct silk with incorporated functionalities such as specific affinity, enzymatic activity, antimicrobial effects, and factors stimulating cell growth.
Mimicking the extracellular matrix to build tissue
Our current research mainly focuses on utilizing silk assembly in combination with biotechnology methods to mimic the in vivo environment, i.e. the extracellular matrix (ECM), to create functional tissue for biomedical applications.
We have developed a method for self-assembly of silk into fibrillar networks with concurrent integration of cells. For this, we use silk proteins functionalized with a cell adhesion motif from the ECM protein fibronectin (FN). Thereby, our functionalized FN-silk makes out a unique 3D culture option within an ECM-like network (Fig. 1A) that allows both cell-cell and cell-matrix interactions, which is lacking in the commonly used hydrogels and scaffold-free spheroids. Moreover, the instant silk assembly allows the direct establishment of cells within frameworks that provide relevant support for the cells, and at the same time allows efficient diffusion of oxygen and nutrients, followed by remodeling with cell migration and inherent ECM production.
We have also developed a method for self-assembly of FN-silk into nanofibrillar membranes (Fig. 1B), mimicking the basement membrane that sits between endothelial tissue and underlying connective tissue. These membranes allow relevant permeation and communication of cells through an elastic membrane of thinness within the cell scale, thereby enabling relevant studies of barrier properties of e.g. vasculature, intestine, skin and lung.
Together, the methods to construct ECM mimics of both interstitial matrix and basement membranes provide valuable tools for building functional tissue units.
Applications for silk-supported tissue constructs
Relevant models of tissue units are getting more and more used within several areas, including disease modeling, toxicology testing, drug development, and precision medicine.
For example, we develop functional in vitro models of breast cancer, to be used for evaluation of drug candidates as well as pre-tests to allow personalized treatment plans.
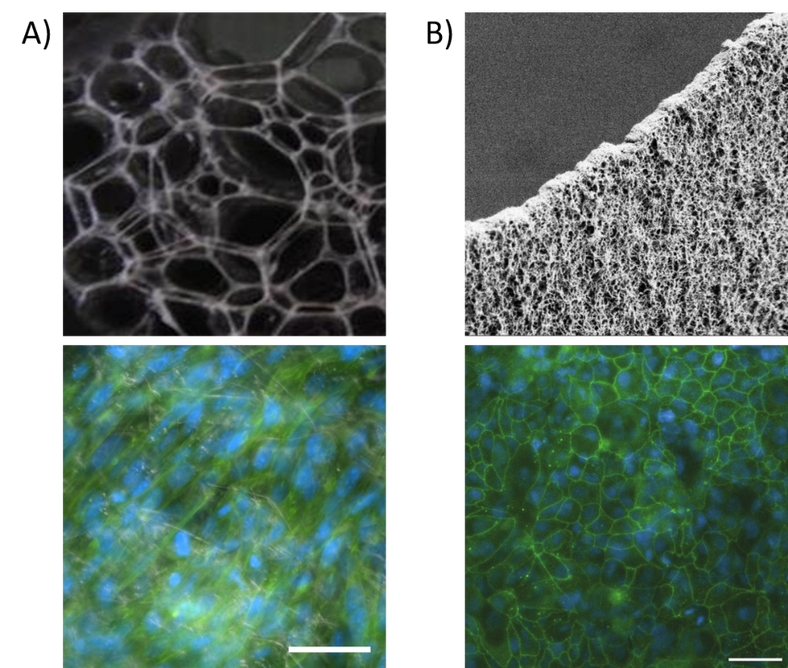
Figure 1. Silk formats for the formation of tissue models. A) FN-silk network without (upper) and with (lower) integrated cells. B) FN-silk membrane without (upper) and with a (lower) confluent layer of cells.
Current group members:
Mona Widhe, Carolina Åstrand, Caterina Collodet, Linnea Gustafsson, Danilo Hirabae de Olivieira, Kelly Blust, Savvini Gkouma, Eirini Ornithopoulou, Astrid Källén
Selected publications:
Oliveira DH, Biler M, Mim C, Enstedt L, Kvick M, Norman P, Linares M, Hedhammar M
Silk Assembly against Hydrophobic Surfaces─Modeling and Imaging of Formation of Nanofibrils
ACS Appl Bio Mater. 2023 Feb 15. https://doi.org/10.1021/acsabm.2c00878
Gustafsson L, Kvick M, Åstrand C, Ponsteen N, Dorka N, Hegrová V, Svanberg S, Horák J, Jansson R, Hedhammar M, van der Wijngaart W.
Scalable Production of Monodisperse Bioactive Spider Silk Nanowires.
Macromol Biosci. 2023 Jan 20. https://doi.org/10.1002/mabi.202200450
Tasiopoulos CP, Gustafsson L, van der Wijngaart W, Hedhammar M
Fibrillar Nanomembranes of Recombinant Spider Silk Protein Support Cell Co-culture in an In Vitro Blood Vessel Wall Model
ACS Biomater Sci Eng. 2021 Jul 12. https://doi.org/10.1021/acsbiomaterials.1c00612
Gustafsson L, Tasiopoulos CP, Jansson, R, Kvick M, Duursma T, Gasser TC, van der Wijngaart W, Hedhammar M
Recombinant Spider Silk Forms Tough and Elastic Nanomembranes that are Protein-Permeable and Support Cell Attachment and Growth
Adv Func Mat 2020 Aug 30. https://doi.org/10.1002/adfm.202002982
Johansson U, Shalaly ND, Hjelm LC, Ria M, Berggren PO, Hedhammar M.
Integration of Primary Endocrine Cells and Supportive Cells Using Functionalized Silk Promotes the Formation of Prevascularized Islet-like Clusters.
ACS Biomater Sci Eng. 2020 Feb 10. https://doi.org/10.1021/acsbiomaterials.9b01573
Kvick M, Tasiopoulos CP, Barth A, Söderberg LD, Lundell F, Hedhammar M
Cyclic Expansion/Compression of the Air-Liquid Interface as a Simple Method to Produce Silk Fibers.
Macromol Biosci. 2021 Jan;21. https://doi.org/10.1002/mabi.202000227
Åstrand C, Chotteau V, Falk A, Hedhammar M
Assembly of FN-silk with laminin-521 to integrate hPSCs into a three-dimensional culture for neural differentiation.
Biomater Sci. 2020 May 7. https://doi.org/10.1039/c9bm01624d
Tasiopoulos CP, Petronis S, Sahlin H, Hedhammar M
Surface Functionalization of PTFE Membranes Intended for Guided Bone Regeneration Using Recombinant Spider Silk
ACS Applied biomaterials. 2020 Jan 3. https://doi.org/10.1021/acsabm.9b00972
Güler R, Thatikonda N, Ghani HA, Hedhammar M, Löfblom J
VEGFR2-Specific Ligands Based on Affibody Molecules Demonstrate Agonistic Effects when Tetrameric in the Soluble Form or Immobilized via Spider Silk.
ACS Biomater Sci Eng. 2019 Dec 9. https://doi.org/10.1021/acsbiomaterials.9b00994
Nilebäck L, Widhe M, Seijsing J, Bysell H, Sharma PK, Hedhammar M.
Bioactive Silk Coatings Reduce the Adhesion of Staphylococcus aureus while Supporting Growth of Osteoblast-like Cells.
ACS Appl Mater Interfaces. 2019 Jul 17. https://doi.org/10.1021/acsami.9b05531
Johansson U, Widhe M, Shalaly ND, Arregui IL, Nilebäck L, Tasiopoulos CP, Åstrand C, Berggren PO, Gasser C, Hedhammar M
Assembly of functionalized silk together with cells to obtain proliferative 3D cultures integrated in a network of ECM-like microfibers.
Sci Rep. 2019 Apr 18. https://doi.org/10.1038/s41598-019-42541-y
Thatikonda, N., Nilebäck, L., Kempe, A., Widhe, M., Hedhammar, M
Bioactivation of Spider Silk with Basic Fibroblast Growth Factor for in Vitro Cell Culture: A Step toward Creation of Artificial ECM
ACS Biomaterials Science and Engineering, 2018 Sep 10. https://doi.org/10.1021/acsbiomaterials.8b00844
Nilebäck L, Arola S, Kvick M, Paananen A, Linder MB, Hedhammar M
Interfacial Behavior of Recombinant Spider Silk Protein Parts Reveals Cues on the Silk Assembly Mechanism.
Langmuir. 2018 Oct 2. https://doi.org/10.1021/acs.langmuir.8b02381
Petrou G, Jansson R, Högqvist M, Erlandsson J, Wågberg L, Hedhammar M, Crouzier T.
Genetically Engineered Mucoadhesive Spider Silk.
Biomacromolecules. 2018 Aug 13. https://doi.org/10.1021/acs.biomac.8b00578
Tasiopoulos CP, Widhe, Hedhammar M
Recombinant spider silk functionalized with a motif from fibronectin mediates cell adhesion and growth on polymeric substrates by entrapping cells during self-assembly.
ACS Applied Materials & Interfaces, 2018 May 2. https://doi.org/10.1021/acsami.8b02647
Gustafsson L, Jansson R, Hedhammar M, van der Wijngaart W c.
Structuring of Functional Spider Silk Wires, Coatings, and Sheets by Self-Assembly on Superhydrophobic Pillar Surfaces.
Adv Mater. 2018 Jan 30. https://doi.org/10.1002/adma.201704325
Mittal N, Jansson R, Widhe M, Benselfelt T, Håkansson KMO, Lundell F, Hedhammar M, Söderberg LD
Ultrastrong and Bioactive Nanostructured Bio-Based Composites.
ACS Nano. 2017 May 23. https://doi.org/10.1021/acsnano.7b02305
Nilebäck L, Hedin J, Widhe M, Floderus LS, Krona A, Bysell H, Hedhammar M
Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study.
Biomacromolecules. 2017 Mar 13. https://doi.org/10.1021/acs.biomac.6b01721
Shalaly ND, Ria M, Johansson U, Åvall K, Berggren PO, Hedhammar M
Silk matrices promote formation of insulin-secreting islet-like clusters.
Biomaterials. 2016 Jun. https://doi.org/10.1016/j.biomaterials.2016.03.006
Widhe M, Shalaly ND, Hedhammar M
A fibronectin mimetic motif improves integrin mediated cell biding to recombinant spider silk matrices.
Biomaterials. 2016 Jan. https://doi.org/10.1016/j.biomaterials.2015.10.013
Johansson U, Ria M, Åvall K, Dekki Shalaly N, Zaitsev SV, Berggren PO, Hedhammar M.
Pancreatic Islet Survival and Engraftment Is Promoted by Culture on Functionalized Spider Silk Matrices.
PLoS One. 2015 Jun 19. https://doi.org/10.1371/journal.pone.0130169
Jansson R, Courtin CM, Sandgren M, Hedhammar M
Rational Design of Spider Silk Materials Genetically Fused with an Enzyme
Advanced Functional Materials 2015 July. https://doi.org/10.1002/adfm.201501833
Jansson R, Thatikonda N, Lindberg D, Rising A, Johansson J, Nygren PÅ, Hedhammar M
Recombinant spider silk genetically functionalized with affinity domains.
Biomacromolecules. 2014 May 12. https://doi.org/10.1021/bm500114e
Renberg B, Andersson-Svahn H, Hedhammar M
Mimicking silk spinning in a microchip.
Sensors and Actuators B: Chemical. 2014 May. https://doi.org/10.1016/j.snb.2014.01.023
Müller C, Hamedi M, Karlsson R, Jansson R, Marcilla R, Hedhammar M , Inganäs O
Woven Electrochemical Transistors on Silk Fibers.
Advanced Materials. 2011 Feb 15. https://doi.org/10.1002/adma.201003601
Askarieh G*, Hedhammar M*, Nordling K, Saenz A, Casals C, Rising A, Johansson J, Knight SD
Self-assembly of spider silk proteins is controlled by a pH-sensitive relay
Nature. 2010 May 13. https://doi.org/10.1038/nature08962
Hedhammar M, Bramfeldt H, Baris T, Widhe M, Askarieh G, Nordling K, Aulock S, Johansson J
Sterilized recombinant spider silk fibers of low pyrogenicity.
Biomacromolecules. 2010 April 12. https://doi.org/10.1021/bm9014039
Courses
Purification of Biomolecules (BB1210), course responsible, examiner