Next Generation Protein Therapeutics
Principal Investigator (PI):
Personnel:
PhD-students (as main supervisor): Sebastian Meister, Charles Dahlsson Leitao
PhD-students (as co-supervisor): Rezan Güler, Linnea Hjelm
PostDocs: Hanna Lindberg, Jonas Persson
Description of present activities:
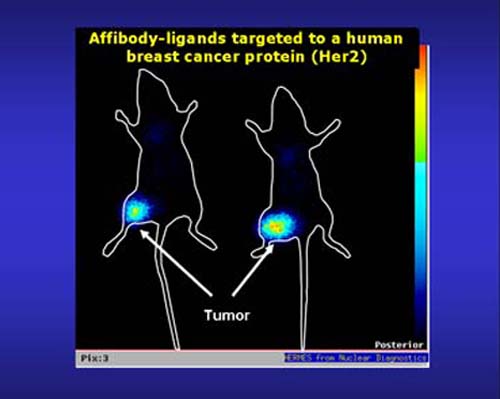
Affibody-mediated biotherapy: A class of small affinity proteins, denoted affibody molecules, has been developed at our department. Affibody molecules are based on a 58-amino acid protein domain, derived from staphylococcal protein A, which has served as scaffold for construction of combinatorial libraries from which affibody binding proteins can be selected to desired target proteins. Their therapeutic potential are investigated in various settings, including tumor targeting for in vivo imaging and therapy applications. Cancer biomarkers such as EGFR, HER2 and HER3 have been used in the context of tumor targeting, and mono- bi- and multispecific formats are being investigated. In addition, the amyloid beta peptide of Alzheimer’s disease (AD) have been used as target for selection of affibody molecules which are subject to preclinical investigations for the prevention of AD symptoms such as memory loss and plaque formation.
Bacterial surface display: The group has during the last 20 years developed a novel host-vector-systems for efficient surface display on food grade staphylococci, e.g. Staphylococcus carnosus. The present focus is to evaluate bacterial display for protein library applications using fluorescence activated cell sorting (FACS) for library screening. The systems are presently evaluated for selection of affinity proteins, i.e. affibody molecules and domain antibodies, using surface-displayed libraries.
Ten selected publications
1. Ståhl, S., Gräslund, T., Eriksson-Karlström, A., Frejd, F.Y., Nygren, P.-Å. and Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends Biotechnol. 35: 691-712(2017). IF 11.1
2. Malm, M., Frejd, F.Y., Ståhl, S. and Löfblom, J. Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds. mAbs 8: 1195-1209 (2016). IF 4.8
3. Bass, T.Z., Rosestedt, M., Mitran, B., Frejd, F.Y., Löfblom, J., Tolmachev, V. Ståhl, S. and Orlova, A. In vivo evaluation of a novel format of a bivalent HER3-targeting and albumin-binding therapeutic affibody construct. Scientific Reports 7:43118. DOI: 10.1038/srep43118 (2017). IF 4.3
4. Wahlberg, E., Rahman, M.M., Lindberg, H., Gunneriusson, E., Schmuck, B., Lendel, C., Sandgren, M., Löfblom, J., Ståhl, S. and Härd, T. Identification of proteins that specifically recognize and bind protofibrillar aggregates of amyloid-ß. Scientific Reports 7:5949 DOI:10.1038/s41598-017-06377-8 (2017). IF 4.3
5. Garousi, J., Anderson, K.G., Dam, J.H., Olsen, B.B., Mitran, B., Orlova, A., Buijs, J., Löfblom, J., Ståhl, S., Thisgaard, H., and Tolmachev, V. The use of radiocobalt as a label improves imaging of EGFR using DOTA-conjugated affibody molecule. Scientific Reports 7:5961. DOI:10.1038/s41598-017-05700-7 (2017). IF 4.3
6. Löfblom, J., Rosenstein, R., Nguyen, M.T., Ståhl, S. and Götz, F. Staphylocoocus carnosus, from starter culture to protein engineering platform. Appl. Microbiol. Biotechnol. DOI 10.1007/s00253-017-8528-6 (2017). IF 3.3.
7. Lindberg, H., Sandersjöö, L., Meister, S., Uhlén, M., Löfblom, J. and Ståhl, S. Flow-cytometric screening of aggregation-inhibitors using a fluorescence-assisted intracellular method. Epub ahead of print: DOI: 10.1002/biot.201600364. Biotech. J. (2017). IF 3.4
8. Cheng, Q., Wållberg, H., Grafström, J., Lu, L., Thorell, J.-O., Hägg-Olofsson, M., Linder, S., Johansson, K., Tegnebratt, T., Arnér, E.S.J., Stone-Elander, S., Martinsson-Ahlzén, H.S. and Ståhl, S. Preclinical PET imaging of EGFR levels: pairing a targeting with a non-targeting Sel-tagged affibody-based tracer to estimate the specific uptake. Eur. J. Nucl. Med. Mol. Imaging, DOI: 10.1186/s13550-016-0213-8 (2016). IF 7.3
9. Fleetwood, F., Güler, R., Gordon, E., Ståhl, S., Claesson-Welsh, L. and Löfblom, J. Novel affinity binders for neutralization of vascular endothelial growth factor (VEGF) signaling. Cell. Molec. Life Sciences. 73: 1671-1683 (2016). IF 5.7
10. Rosestedt, M., Andersson, K.G., Mitran, B., Rinne, S.S., Tolmachev, V., Löfblom, J., Orlova, A., and Ståhl, S. Evaluation of a radiocobalt-labelled affibody molecule for imaging of human epidermal growth factor receptor 3 (HER3) expression. Internat. J. Oncol. DOI: 10.3892/ijo.2017.4152 (2017). IF 3.1

