Continuous Bioprocess
At the Swedish competence center AdBIOPRO, research specialists in both upstream and downstream processing join forces and have successfully achieved scaling up in a continuous process at a test center in Sweden.
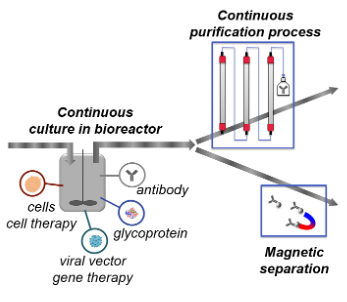
Integrated continuous bioprocess
Biopharmaceuticals have become the best selling drugs in terms of revenues, e.g. with monoclonal antibody sales, compared to small molecules. Beyond the supremacy of monoclonal antibodies, the actual trends are going towards growing pipelines of biopharmaceuticals, more complex protein drugs but also cell-based emerging alternative therapeutic approaches such as gene and cell therapy.
In the domain of biopharmaceutical manufacturing, the legacy is fed-batch process (i.e. batch process fed with time) followed by several batch purification steps, e.g. chromatography steps, supported by in house industrial technology platforms. However the field is rapidly evolving by incorporating novel technologies to meet the challenges brought by the new trends and a constant need of competitiveness. In this perspective, continuous processes have recently received a very high interest. Integrated continuous bioprocesses consist of perfusion culture where medium is steadily added and harvest is removed to be processed in a purification process made of continuous consecutive steps.
Continuous bioprocesses have small footprint in comparison with legacy batch processes. They can be produced with disposable equipment, which enables reproducing the manufacturing according to the demand, low or very high. It is therefore an excellent strategy to produce low volumes in normal case and be able to quickly multiply the production world-wide in case of very high need such as pandemies. Continuous process ensures as well very good control of the product quality. The Health Authorities encourage usage of continuous bioprocessing, among others, due to the good control of product quality.