MRI with high-intensity ultrasound (MRI-HIFU)
Technology description
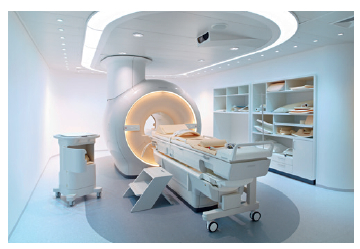
The MRI scanner (Philips Ingenia 3T) has fast gradients (80 mT/m peak amplitude , or 40 mT/m peak amplitude combined with a slew rate of 200 mT/m/ms) and the ability to reconstruct 4-D blood flow in the heart and blood vessels. The system automatically selects the coil elements for optimized signal. Digitized coils and a fiber optic cable provide an enhanced SNR and high dynamic range (max 187 dB). The Xtend imaging space feature provides increased homogeneity and image quality, and enables larger field of view (FOV), up to 70 cm. The MRI system is designed in such a way that channels are determined by the coil rather than the system. This enables expansion of capabilities with new coil design avoiding major system upgrades.
Phillips’ 3T MRI machine is equipped with the High Intensity Focused Ultrasound (HIFU) for therapeutic application. This unit is inherently integrated into the MRI scanner, offering improved patent safety for both the feedback of the temperature elevation and overall therapy planning and monitoring of the effect.
For research purposes, the scanner is equipped with:
Parallel RF excitation
The MRI scanner has a technology to calibrate the transmitter field (B) specifically for each patient (RF shimming) to enhance image uniformity and consistency. This eliminates the effect of the dielectric shading.
By using multiple RF sources, it is possible to reduce local Specific Absorption Rate (SAR) and take advantage of optimized RF management to provide faster scanning.
MRI-guided HIFU
The HIFU equipment is integrated in the scanner, and MRI-guided HIFU can be performed with continuous temperature monitoring through MR thermometry.
High Order Shim
When the inhomogeneity in the patient is considered to be significant at a field strength of 3T, the “high order shim” is required.
MultiTransmit 4D
MultiTransmit parallel RF transmission enhances signal and image uniformity and consistency even during real time cardiac applications. Using two independent RF sources, amplifiers and receivers enables patient adaptive shimming.
Diffusion imaging
The scanner is equipped with diffusion-weighted imaging (DWI) as well as diffusion-tensor imaging (DTI), which is particularly useful in neuroimaging and metastasis screening.
IntelliSpace Portal - Tumor tracking
Allows simultaneous multimodality viewing and assessment of tumor size progression, which may be useful as an alternative to PET-CT in whole body oncology staging and follow-up.
Available coils:
| Coil Name |
Application area |
Coverage (channels) |
| dS TotalSpine |
Total spine, C‐Spine, T‐Spine, L‐Spine |
90 cm coverage using 44 channels |
| dS HeadSpine |
Head, Brain, Neuro, Total spine, C-Spine, T-Spine, L-Spine |
30 cm(15 channels) Head 90 cm (51 channels) Neuro |
| dS HeadNeckSpine |
NeuroVascular, Head, Brain, Pediatric,Total Neuro, Total spine, C‐Spine, T‐Spine, L‐Spine |
45 cm (20 channels) Head‐Neck 90 cm (52 channels) Total Neuro |
| dS WholeBody |
Whole body, Peripheral‐vascular, Torso, Chest, Pelvis, Heart |
200 cm (108 channels) |
| dS Flex |
Shoulder, Foot, Ankle, Knee, Pediatric |
15 cm (6 channels) |
| dS Knee |
Knee, extremities |
20 cm (16 channels) |
| dS Small Extr |
Elbow, Arm, Extremities |
20 cm (8 channels) |
Scanning Tools
Scanning tools available in the MRI scanner contains imaging methods for the assessment of morphology of all anatomical areas including brain and spine, muskuloskeletal system, breast, cardiac, and various blood vessels with or without contrast agents.
The following workflow features are available for all clinical anatomies:
“ExamCards” are designed for automated scanning and processing of patient studies.
“SENSE” feature is used for parallel imaging methods for fast scan times, high resolution or to reduce susceptibility artifacts.
“CLEAR” feature enhance the signal uniformity based on coil‐sensitivity and on patient loading.
“PicturePlus” feature improves appearance of images through edge enhancement and smoothing. Provides full control over all enhancement parameters, which can be applied automatically post‐acquisition or as a post‐processing option.
Clinical impact
The 3T MRI scanner is intended to be used for advanced research purposes in collaboration between KTH, Karolinska Institutet and Stockholm County Council based on the donation that KTH received from Kerstin and Rune Jonasson. Moreover, it is used for clinical purposes in collaboration with the Karolinska University Hospital Huddinge.
Integration of HIFU and flow measurement in the MRI camera with a unified interface is essential for patient safety, since every solution that enables data to be transferred between devices without rigorous standardized flows can result in a risk of mistakes (errors in manual calculations, risk of confusion of patient identity and the left-right, etc.).
Manufacture web page of the equipment

More news on Lego MRI scanner…
www.bbc.com/news/uk-england-berkshire-26438170
_
Procedure
After approval of an MRI project, but before acquiring image data, users need to contact the MRI physicists at the hospital Sven Petersson (sven.petersson@sll.se) for safety instructions and Karin Telin (karin.telin@sll.se) about getting access to the premises.
In addition to the ethical approval, researchers will have to arrange for competent review and handling of incidental findings (see Avtal datahantering MRT KTH-SLL 200317 signerad.pdf (pdf 2.5 MB) ).
Image data needs to be stored in the hospital's PACS for potential clinical needs in the future. If the researchers want to make use of the hospital's service for access to image data (rather than downloading anonymized image data directly from the scanner), they will have to follow the hospital's routines for handling of patient data according to the following documents:
Begäran_Bilddatautlämnande_K_v1_200525.pdf (pdf 336 kB)
Bilaga3_0 utlämnande av journaluppgifter200214.pdf (pdf 199 kB)
Bilaga3_1 utlämnande till myndigheter m m 200214_1.pdf (pdf 158 kB)
Bilaga3_2 delegering av ansvar för menprövning200214.docx (docx 121 kB)
Bilaga3_3 begäran om uttag av patientdata för forskning.docx (docx 73 kB)
Bilaga3_4 beslutsmall utlämnande av data till forskare.docx (docx 51 kB)
As the information in these documents is subject to change, it should be checked with hospital B&F administration (<catrine.wetterqvist@sll.se>) for updates.
Contact person
For more information please contact the Jonassons Medical Imaging Centrum.
