James Gardner
Associate professor
Details
Researcher
About me
Research in the Gardner Group
The photochemistry and photophysics of molecules at interfaces have been an overarching research theme in the Gardner Group. Of particular interest is understanding the energetics, kinetics, and conditions that promote bond formation and charge transfer chemistry for solar energy conversion. As well, a strong synthetic expertise allows us to tailor molecules and materials to study match our experimental needs.
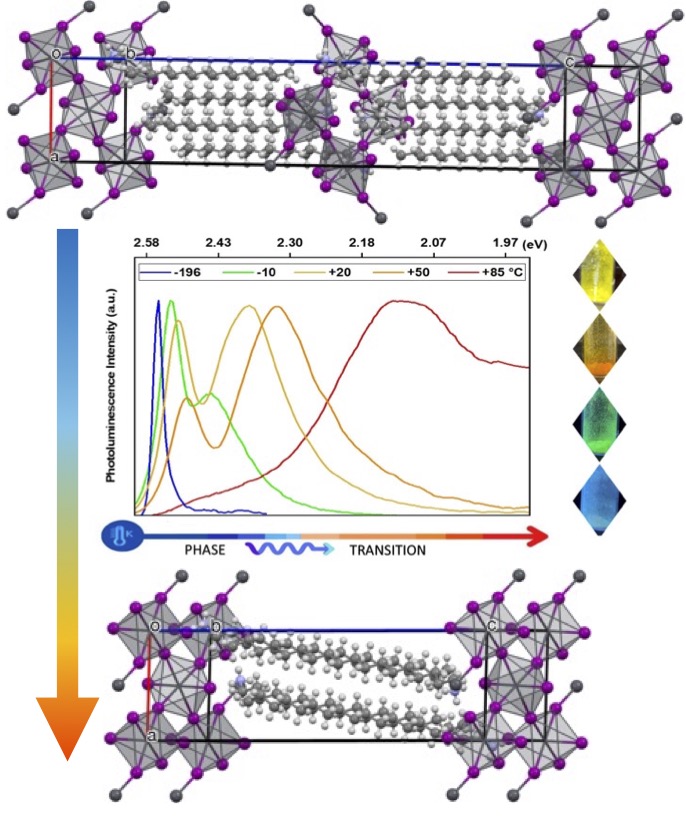
To understand interfacial charge transfer in perovskite solar cells, the Gardner lab applies its expertise to the synthesis, photophysics, and photochemistry of perovskites. An overarching goal of these studies is to make safe and stable materials for high efficiency solar cells.
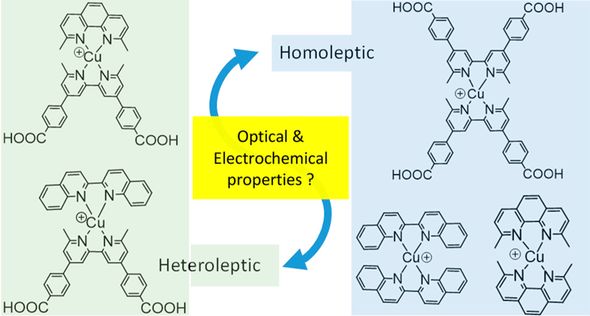
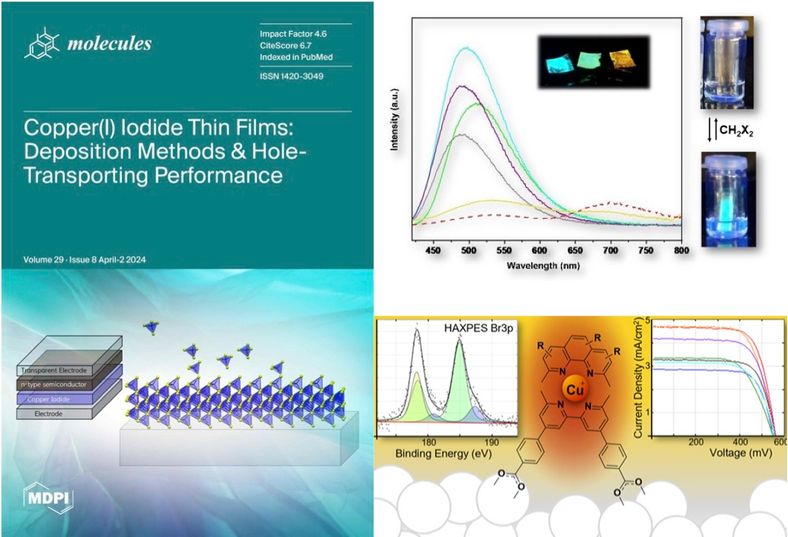
Our studies of molecular materials have focused on copper compounds for use in dye-sensitized solar cells , sensors, and optoelectronic applications.
Copper coordination complexes may one day supplant ruthenium complexes as the go-to photochemistry molecules. These molecules have similarly long excited state lifetimes, rely on an abundant metal, and have comparatively direct syntheses. However, these molecules are hampered by their relative (photo)chemical instability. A deeper understanding of the bonding in copper coordination may lead to more robust molecules and less expensive solar cells.
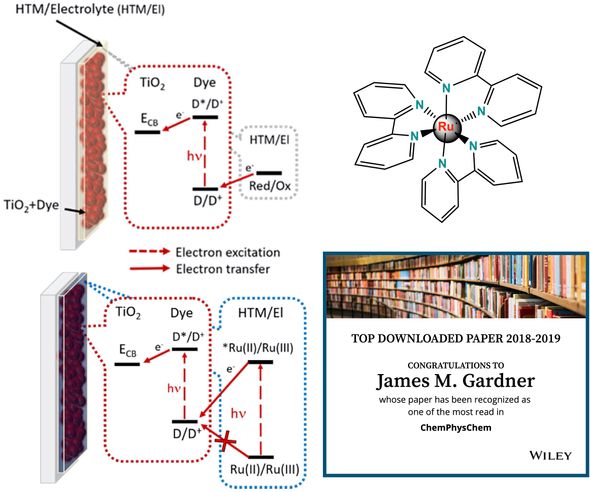
The Gardner lab has studied ruthenium coordination complexes for their potential use in tandem solar cells made from strictly molecular materials. Ruthenium complexes are well known for their photostability and tunable redox chemistry and comparatively long-lived excited states.
Education and Experience
Bachelor of Science in Chemistry and Environmental Science (Tulane University, 2004)
Ph.D. in Chemistry (The Johns Hopkins University, 2009)
During his post-doctoral research at Uppsala University (2009-2011), Dr. Gardner explored interfacial charge transfer chemistry by laser-based time-resolved spectroscopies.
James joined the faculty of KTH in 2012 as the Assistant Professor of Photoelectrochemistry.
In 2016, James received the title of Docent and was promoted to tenured Associate Professor.
From 2018 to 2024, Dr. Gardner was the Division Head for Applied Physical Chemistry at KTH's Department of Chemistry. Those same years, James was Deputy Head of the Center for Molecular Devices (CMD). The CMD is a collaborative research environment between KTH and Uppsala University that focuses on all aspects of emerging solar cell technologies. Dr. Gardner is currently an editor at the journalsEnergies (since 2020) andNanoenergy Advances (since 2022).
In the News
Moisture tolerant perovskite solar cells: link
James was awarded the element Tantalum from IUPAC for the Periodic Table of Younger Chemists: link
KTH News:
1) Meeting with KTH's Energy Platform
2) Possible solution to flaw in promising solar material
3) Solar cell performance improves with ion-conducting polymer
Courses
Advanced Inorganic Chemistry (CK2020), course responsible, examiner
Batteries (CK206V), teacher
Chemical Equilibrium (CK1280), teacher, course responsible, examiner
Chemical Equilibrium (CK1285), course responsible, examiner
Chemical Principles for Sustainability (KD1000), teacher
Degree Project in Chemical Engineering and Technology, First Cycle (KH138X), examiner
Degree Project in Chemistry, Second Cycle (KD200X), examiner
Degree Project in Fibre and Polymer Technology, Second Cycle (KF200X), examiner
Introductory Chemistry (KD1020), teacher
Materials Chemistry and Properties (CK1165), teacher
Project in Chemistry (KD2910), examiner
Project in Chemistry (KD2920), examiner
Project in Chemistry (KD2905), examiner