RAPP-ID: Development of rapid point-of-care test platforms for infectious diseases
Excellent care for people with suspected infections involves rapid diagnosis and treatment. For instance, administering the correct antibiotic as soon as possible to patients with blood infections, dramatically improves their chances of survival. Equally so, using antibiotics when they do not benefit patients expose them unnecessarily to side effects and potential antibiotic resistance. In this modern age, we still do not have the technology available that can quickly diagnose what kind of infection and what treatment is needed. Even the best of the currently available diagnostic methods are too slow to help clinicians.

Excellent care for people with suspected infections involves rapid diagnosis and treatment. For instance, administering the correct antibiotic as soon as possible to patients with blood infections, dramatically improves their chances of survival. Equally so, using antibiotics when they do not benefit patients expose them unnecessarily to side effects and potential antibiotic resistance. In this modern age, we still do not have the technology available that can quickly diagnose what kind of infection and what treatment is needed. Even the best of the currently available diagnostic methods are too slow to help clinicians.
- Blood infections,
- Lower respiratory tract Infections, including community-acquired pneumonia and ventilator-associated pneumonia,
- Tuberculosis
Detection of bacteria, fungi and antibiotic resistance will mainly involve Nucleic Acid tests, whereas viral and markers of infection detection will mainly involve selective immunobinding with a probe or with a sensor surface.
The diagnostic tests will consist of four functional modules: sample collection and interfacing; upconcentration and extraction; signal and/or sample amplification; and detection. RAPP-ID will integrate the modules required for each disease/syndrome in a matrix to be used with an instrument that reads the results, also developed within the project. The diagnostic platform will be validated on well-characterised clinical samples and compared with the best reference standards and other currently available diagnostic tests.
The RAPP-ID consortium draws its expertise from across Europe and includes 10 academic partners, 4 SMEs and 5 EFPIA companies who will work together over a 5-year period.
MST contribution to RAPP-ID
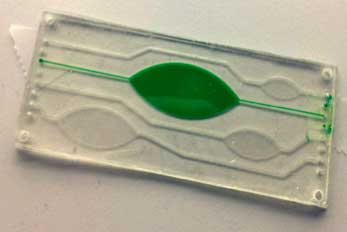
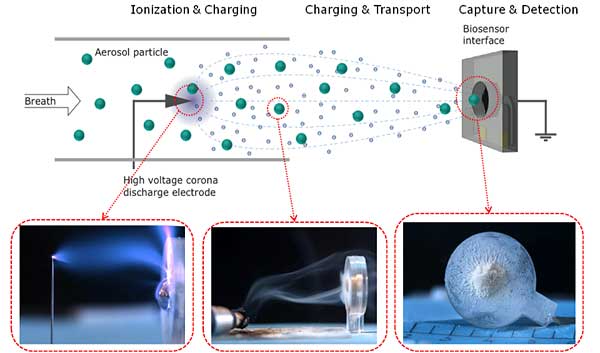
In RAPP-ID MST brings its expertise in sample collection via electrohydrodynamic transport and transduction via QCMs, both developed in the RPA project and the novel polymer concept of off stoichiometry thiol-enes (OSTE).
The use of the OSTE family of polymers allows for research prototypes to be brought into medium to high volume production devices at an unprecedented speed, thanks to the similarities between specially formulated OSTE polymers and injection molding plastics such as COC (Cyclic Olefin Copolymer) and Polycarbonate in terms of mechanical and surface properties
RAPP-ID website
Project members
Project sponsor
- European commission, 7th framework programme
- EFPIA partners
Publications related to this project
- Aerosol sampling using an electrostatic precipitator integrated with a microfluidic interface
- Integrating Biosensors for Air Monitoring and Breath-Based Diagnostics
- From Macro to Nano : Electrokinetic Transport and Surface Control
- SURFACE ENERGY MICROPATTERN INHERITANCE FROM MOLD TO REPLICA
- Rapid mold-free manufacturing of microfluidic devices with robust and spatially directed surface modifications
- Rapid ultra-sensitive detection of influenza A nucleoproteins using a microfluidic nonlinear acoustic sensor
- Screening of antibodies for the development of a fast and sensitivie influenza : A nucleoprotein detection on a nonlinear acoustic sensor
- ROBUST MICRODEVICE MANUFACTURING BY DIRECT LITHOGRAPHY AND ADHESIVE-FREE BONDING OF OFF-STOICHIOMETRY THIOL-ENE-EPOXY (OSTE+) POLYMER
- Integration of a QCM with an OSTE cartridge
- Simple integration of a biosensor with an OSTE polymer cartridge by low temperature dry bonding
- ONE STEP INTEGRATION OF GOLD COATED SENSORS WITH OSTE POLYMER CARTRIDGES BY LOW TEMPERATURE DRY BONDING
- Lab-on-a-chip microsystems for point-of-care diagnostics
- Microfluidic systems for point-of-care diagnostics
- Electrohydrodynamic Enhanced Transport and Trapping of Airborne Particles to a Microfluidic Air-Liquid Interface
- Microsystems for Airborne Sample Detection
- ELECTROHYDRODYNAMIC ENHANCED TRANSPORT AND TRAPPING OF AIRBORNE PARTICLES TO A MICROFLUIDIC AIR-LIQUID INTERFACE
