Research
The aim of our research has been to develop new selective methods for organic synthesis enabling the preparation of organic compounds with well-defined three-dimensional structures. To achieve this, we have designed new selective catalysts based on transition metals, which we have applied in asymmetric synthesis, and developed new enantioselective catalytic processes.
Projects include:
Lewis acid-Lewis base catalyzed reactions:
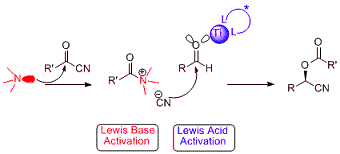
Highly enantioenriched acylated cyanohydrins were obtained in one step in high yields via our Lewis acid - Lewis base catalyzed cyanation of aldehydes using ketonitriles. The process is fairly general in that a large variety of aldehydes and ketonitriles can be used. The products, which are obtained with perfect atom economy, serve as important synthetic building blocks and have in themselves useful applications as e g environmentally friendly insecticides and herbicides. Publications: S. Lundgren, E. Wingstrand, M. Penhoat, C. Moberg, J. Am. Chem. Soc. 2005, 127, 11592; S. Lundgren, E. Wingstrand, C. Moberg, Adv. Synth. Catal. 2007, 349, 364; E. Wingstrand, L. Fei, S. Lundgren, M. Penhoat, C. Moberg, Chem. Oggi 2007, Suppl to Vol 25, no 5, 14; A. Laurell Nash, R. Hertzberg, Y.-Q. Wen, B. Dahlgren, T. Brinck, C. Moberg, Chem. Eur. J.2016, 22, 3821.
Minor Enantiomer Recycling:
Reviews: C. Moberg, Acc. Chem. Res. 1016, 49, 2736; C. Moberg, Pure&Appl. Chem., 2016, 88, 309. Other publications: E. Wingstrand, A. Laurell, L. Fransson, K. Hult, C. Moberg, Chem. Eur. J.2009, 15, 12107; L. Fransson, C. Moberg, ChemCatChem.2010, 2, 1523; L. Fransson, A. Laurell, K. Widyan, E. Wingstrand, K. Hult, C. Moberg, ChemCatChem.2010, 2, 683; A. Laurell Nash, K. Widyan, C. Moberg, ChemCatChem.2014, 6, 3314; R. Hertzberg, G. Montreal Santiago, C. Moberg, J. Org. Chem.2015,80, 2937; R. Hertzberg, C. Moberg, Synthesis2016,48, 3175; C. Moberg, Acc. Chem. Res. 2016, 49, 2736; C. Margarita, A. Laurell Nash, D. A. Ahlstrand, M. S. G. Ahlquist, O. F. Wendt, L. Fransson, C. Moberg, ChemSystemsChem 2024, 6, e202300045.
Secondary interactions: M…H-O hydrogen bond:
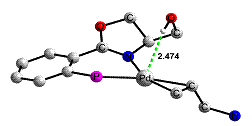
During studies of palladium catalyzed asymmetric allylic alkylations we found evidence for a hydrogen bond where Pd(0) serves as the proton acceptor. The presence of this hydrogen bond has major consequences for the stereochemistry of the catalytic process since the conformation of the ligand is affected by the interaction. For this reason ligands with suitably situated hydroxy groups and their O-alkylated analogues exhibit different stereochemical preferences. This is the first example where this type of interaction has consequences for the stereochemistry of a catalytic reaction. DFT computations verified our conclusions: K. Hallman, T. Wondimagegn, A. Frölander, M. Svensson, C. Moberg, Proc. Natl. Acad. Sci. U.S.A.2004,101, 5400; A. Frölander, S. Lutsenko, T. Privalov, C. Moberg, J. Org. Chem.2005, 70, 9882.
The Role of Symmetry in Asymmetric Catalysis:

We have explored the influence of symmetry, in particular C3symmetry, in asymmetric reactions. Major experimental studies are based on chiral TREN (tris(2-aminoethyl)amine) derivatives, obtained via our ring opening of chiral aziridines by ammonia. Conceptual surveys are found in: C. Moberg, Angew. Chem. Int. Ed. 1998, 37, 248; C. Moberg, Isr. J. Chem. 2012, 52, 653. Other publications: C. Moberg, Angew. Chem.,Int. Ed.2006, 45, 4721; E. Brulé, Y. Pei, F. Lake, F. Rahm, C. Moberg, Mendeleev Commun.2004, 14, 276; Y. Pei, K. Brade, E. Brulé, L. Hagberg, F. Lake. Moberg, C., Eur. J. Org. Chem. 2005, 2835; C. Moberg, Isr. J. Chem. 2012, 52, 653; C. Moberg, Bull. Chem. Soc. Jpn, 2021, 94, 558.
The performance of P,N-ligands with pseudo-C2and pseudo-Cssymmetry, the former “sterically C2-symmetric” but electronically asymmetric, the latter sterically meso but electronically asymmetric, in catalytic applications have led to valuable insight into the influence of electronic and steric properties in palladium catalyzed allylic alkylations: J.-L. Vasse, R. Stranne, R. Zalubovskis, C. Gayet. C. Moberg, J. Org. Chem. 2003, 68, 3258. J.-L. Vasse, R. Stranne, R. Zalubovskis, C. Gayet. C. Moberg, J. Org. Chem. 2003,68, 3258.
Self adaptable catalysts:

The discovery that ligands with pseudo-C2symmetry result in higher selectivity for substrates giving rise to syn-syn-allyl complexes, whereas ligands with pseudo-Cs symmetry are preferred for reactions involving anti-anti-allyl complexes in Pd-catalyzed asymmetric alkylations led us to design and study a new type of catalysts capable of adapting their structure to the reacting substrate: R. Zalubovskis, E. Fjellander, Z. Szabó, C. Moberg, Eur. J. Org. Chem. 2007, 108; R. Zalubovskis, A. Bouet, E. Fjellander, S. Constant, D. Linder, J. Lacour, T. Privalov, C. Moberg, J. Am. Chem. Soc. 2008, 130, 1845; R. Bellini,M. Magre, M. Biosca, P.-O. Norrby, M. Diéguez, O. Pamies, C. Moberg, ACS Catalysis2016, 6, 1701; L. Theveau, R. Bellini, P. Dydio, Z. Szabo, A. van der Werf, R. A. Sander, J. N. H. Reek, C. Moberg, Organometallics2016, 35, 1956. M. Diguez, O. Pamiès, C. Moberg, Acc. Chem. Res. 2021, 54, 3252; M. Diguez, O. Pamiès, C. Moberg, Acc. Chem. Res. 2021, 54, 3252.
Element-element additions:
We have contributed with the first enantioselective element-element addition to 1,3-dienes and the first example of allene formation via element-element addition to 1,3-enynes. Two surveys of the field have been published together with prof I Beletskaya: I. Beletskaya and C. Moberg, Chem. Rev.1999, 99, 3435; I. Beletskaya, C. Moberg, Chem. Rev. 2006, 106, 2320. Other publications: M. Gerdin, C. Moberg, Adv. Synth. Catal.2005, 347, 749; M. Gerdin, C. Moberg, Org. Lett. 2006, 8, 2929; C. Lüken, C. Moberg, Org. Lett.2008, 10, 2505; G. Durieux, M. Gerdin, C. Moberg, A, Jutand, Eur. J. Inorg. Chem. 2008,27, 4236; H. Zhou, C.Moberg, J. Am. Chem. Soc. 2012, 134, 15992; Y. Xiao, C. Moberg, Org. Lett. 2016, 18, 308; E. Li, H. Zhou, V. Östlund, New J. Chem. 2016, 40,6340. C. Moberg, Synthesis, 2020, 52, 3129.
Supported catalysts:
We have developed several efficient methods for covalent attachment of ligands to solid supports, including the attachment of chiral ligands to planar and structured silicon surfaces. Several of the polymeric ligands have been shown to behave successfully in catalytic applications, allowing recovery and reuse of the chiral ligands up to 30 times and of the entire metal complex up to four times without loss in selectivity or reactivity. Ligands and metal complexes have also been immobilized on dendrimers and flat as well as structured silicon surfaces. O. Belda, S. Lundgren, C. Moberg, Org. Lett. 2003, 5, 2275; M. Tilliet, S. Lundgren, C. Moberg, V. Levacher, Adv. Synth. Catal. 2007, 349, 364; S. Lundgren, A. Russom, C. Jönsson, G. Stemme, S. J. Haswell, H. Andersson, C. Moberg, Special Publication - Royal Society of Chemistry 2004, 296 (Micro Total Analysis Systems 2004, Volume 1), 445;H. Andersson, C. Jönsson, C. Moberg, G. Stemme, Sensors and Actuators B, 2001, 79, 78; H. Andersson, C. Jönsson, C. Moberg, G. Stemme, Electrophoresis2001, 22, 3876; H. Andersson, C. Jönsson, C. Moberg. G. Stemme, Talanta 2002, 56, 301; C. Jönsson, K. Hallman, H. Andersson, G. Stemme, M. Malkoch, E. Malmström, A. Hult, C. Moberg, Bioorg. Med. Lett. 2002, 12, 1857; K. Hallman, M. Malkoch, S. Lutsenko, E. Malmström, A. Hult, C. Moberg, J. Org. Chem. 2002, 67, 8197.
Micro reactors, high throughput screening: synthesis and analysis:
In order to enable efficient screening of reactions and reaction conditions we have used micro reactors for the optimization of asymmetric metal catalyzed reactions. We have for example successfully studied the influence of the structure of Lewis bases in Lewis acid-Lewis base catalyzed additions of ketonitriles to aldehydes using microreactor technology. S. Lundgren, H. Ihre, C. Moberg, Archivoc,2008, 6, 73.
Enzymatic determination of enantioselectivity and conversion in catalytic reactions:
We have developed an enzymatic method (jointly with prof K. Hult and coworkers) for the simultaneous analysis of yield and enantioselectivity in the synthesis of acylated cyanohydrins: A, Hamberg, S. Lundgren, M. Penhoat, C. Moberg, K. Hult, J. Am. Chem. Soc., 2006, 128, 2234; A. Hamberg, S. Lundgren, E. Wingstrand, C. Moberg, K. Hult, Chem Eur J, 2007, 13, 4334.
