ADVANCED LIGHT MICROSCOPY LAB SciLifeLab
The mission of the ALM laboratory at SciLifeLab is to give access and expert support in state-of-the-art fluorescence microscopy techniques. Ever since the laboratory was started in 2010 a strong focus has been on super-resolution microscopy. In 2017, two new staff scientists were recruited to broaden the services to also include light sheet microscopy and advanced FCS analysis.

The introduction of Light sheet microscopy at ALM has attracted several new research groups with a need to image larger samples, model organisms, and also for studies of cleared tissue samples. In parallel to the introduction of new imaging techniques the laboratoryhas developed sample preparation protocols and gives now advanced support that includes clearing and expansion techniques. Single molecule dynamics studies in correlation spectroscopy have been established as a core technology in the facility and there is a continuously increased use and broadened interest for those techniques.
Arriving at the lab,
Hjalmar Brismar
told us about the project – Science for Life Laboratory.
‘We applied for the initial construction of SciLifeLab in 2009, as a joint project between KTH, Karolinska Institute, and Stockholm University”.

SciLifeLab is now a national infrastructure since 2013 with main laboratories in Stockholm and Uppsala but also specialized labs at Umeå, Gothenburg, Lund and Linköping Universities. KTH is the coordinator and main responsible for SciLifeLab. In fact, SciLifeLab and Campus Solna is KTH's second largest campus and house almost 50 KTH research groups from the SCI, CBH and EECS schools.
The focus of our visit today is on the advanced light microscopy facility (ALM). This is an infrastructure that has been constructed based on the long experience KTH has in development of microscopy techniques. Development of microscopy is a core scientific activity in the Biophysics unit. It is driven by the needs in cell biology and cellular biophysics to study fine details in cells with high specificity and sensitivity.
”What is unique about this facility is not only the techniques that are available but also that it is a national and international flagship laboratory for superresolution imaging. We are the only lab placed in the Nordic countries that can do all those technologies in one place, and we are part of the European infrastructure EuroBioimaging. What makes this unit special is not only the machines but also the staff scientists operating them, who are the best ones in the world to support the use of the microscopes available.’
The main staff members are Prof. Hjalmar Brismar (Facility Director), Docent Hans Blom (Head of Facility), Ana Agostinho, Stefan Wennmalm, and Steven Edwards (Staff scientists).
Equipment
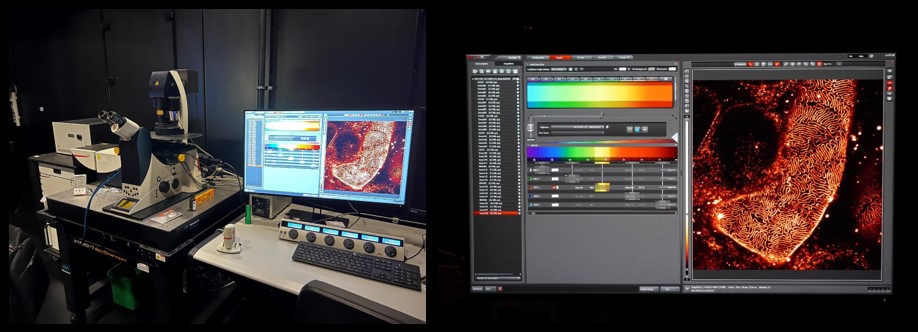
‘We have a super resolution microscope, which goes beyond the diffraction limit - the usual limit for light microscopes. This certain microscope uses the stimulated emission depletion (STED) microscopy method, which was awarded a Nobel a couple of years ago. With this machine, we can go down to at least 50 nanometers. What you see on the screen is the fine structure in the kidney, the filtration slit in the fine capillaries. In the past, that was something that one could only study with electron microscopy. However, we have developed techniques that make it possible to see it in the light microscope. Moreover, we have further developed techniques that allow us to have the same result using a normal microscope, by creating an enlarged physical copy of the sample. The combination of the sample expansion and the super resolution techniques can lead to impressive results that even exceed previous super resolution microscopes' limits.’

Ana Agostinho demonstrated the use of a microscope that uses a variant of super resolution techniques that give about two times higher resolution in all directions, compared to normal microscopy. This gives scientists eight times more information about their sample.
In the picture we can see mouse spermatocytes (in blue) when first acquired, compared with the result after careful processing using super resolution techniques.
Stefan Wennmalm explained Fluorescence Correlation Spectroscopy – single molecule spectroscopy measurement and analysis to evaluate interaction, aggregation, mobility, and dynamics. In particular, he showed us how, by using fluorescence in plasma membranes, one can study the movement of monomers and dimers of proteins as well as their relative distance to each other.

After these impressive demonstrations, we asked Hjalmar about the future plans of the lab.
’Well we are developing all the time. The ambition of the ALM infrastructure is to continuously develop the microscopy techniques and the staff competence so that we maintain our position as a unique laboratory at the technical forefront of microscopy.”
In that direction, the latest news is that we received a nice VR grant (15 million) that will allow us to get a new version of the microscope demonstrated by Ana Agostinho, using a newer version of the Lattice SIM technique.’

Moving on to the second dark lab room of the facility, the light sheet laboratory.
Hjalmar described the experimental setup of the light sheet method, where the detection sample is sitting in the chamber, in a fluid, in the centre and two objectives on the sides are projecting light into the sample. Light is coming from the sides, producing a light sheet, and then the objective at the back is used to take pictures of the light sheet, so one gets to see the very thin layer illuminated, and then move that layer, back and forth, recording a 3D image of the sample. The sample can also be rotated to result in a 3D rendering. One can study, for example, the zebrafish and follow their neuron development. The zebrafish is a good model organism that has a lot of similarities to humans since it is a vertebrate. Therefore, one can study various phenomena during development in zebrafish, and then translate that to how it works in humans.
Hjalmar explained that there are plans to have it rebuilt in the next half a year to the general serial version, the one commercially available now. It is based on Betzig’s idea and his prototype, and he got the Nobel Prize in 2014 on super resolution microscopy . The lattice light sheet microscopy is better than the previous techniques in many ways. It is a perfect combination of high resolution, very low phototoxicity - that is very low illumination power, so we don't disturb the material that much. It also has a very high recording frequency so we can record fast events in living cells in three dimensions or four dimensions, even over long periods.
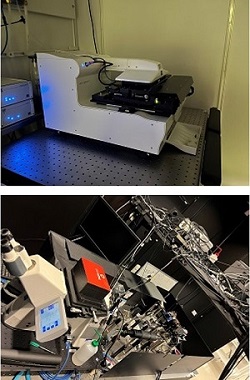
Moreover, we saw the labs where microscopes and sample technique preparations are developed. Additionally, we visited the newly developed multi focus microscope with SIM, structured illumination microscopy. Combining super resolution with the patterns and simultaneously recording from different focus levels in the sample, we get super resolution in 3 dimensions at once.
Lastly, we visited the cell culture and preparation lab. There one can see animal tissue for example, which is especially prepared according to the microscopy technique it will undergo.
Later, we visited Hjalmar’s office where we saw some awesome photographs taken by the microscopes we had just visited.

Text: Danai Deligeorgaki and Elina Charatsidou
