Potential treatment may prevent brain damage in premature babies
A treatment that could protect premature babies from brain damage showed promise in a recent study. Using a first-of-its-kind prenatal brain model created with human cells researchers observed new details about the effects of cerebral hemorrhages on stem cells during premature birth. And they successfully tested an antidote that reduced the damage.
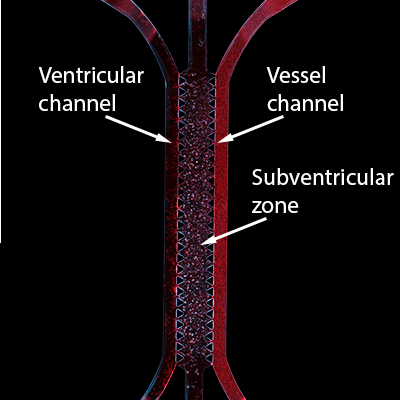
Publishing in Advanced Science , the researchers identified how neural stem cells in preterm infants are damaged as a result of a cerebral hemorrhage. A team from KTH Royal Institute of Technology, Karolinska Institutet, and Lund and Malmö Universities collaborated on the study.
The study shows that as red blood cells seep into the brain’s subventricular zone (SVZ) and break down, levels of the immune response messenger protein interleukin-1 (IL-1) become elevated. These proteins send strong signals that direct neural stem cells to stop acting like stem cells, says Professor Anna Herland , senior lecturer at the AIMES research center at KTH Royal Institute of Technology and Karolinska Institutet.
“Instead of remaining flexible and ready to grow into different types of brain cells, the stem cells start changing too early or stop growing altogether,” Herland says.
Complication at pre-term birth
Intraventricular hemorrhage (IVH) is a frequent and severe neurologic complication of preterm birth. When red blood cells enter the brain, the plasma proteins and antioxidants that protect them in the bloodstream become overwhelmed by pressure and stress. Blood cells rupture and release inflammation-triggering components such as hemoglobin, which triggers pro-inflammatory proteins associated with immune response, such as interleukin-1.
“Blood and its degradation products cause a strong inflammatory response in brain support cells, glia cells, that are meant to protect and nourish the brain and repair damage,” Herland says.
Medical science has previously experimented on animals to research the effects of red blood cell lysate, but the model developed in Sweden breaks new ground. It enables these effects to be studied for the first time in a system that closely mimics mechanisms of the human brain. In collaboration with researchers at Ege University in Turkey and Harvard University in the U.S., the team built its model with living lab-grown human brain cells derived from stem cells, which enables examination of responses in the vulnerable brains of premature babies.
Complex in vitro model
The model provided a unique platform for successfully testing an antidote: an IL1 antagonist, which was shown to suppress levels of interleukin-1, providing partial protection to the stem cells.
The impact of cerebrospinal fluid (CSF) from patients with hemorrhage was studied separately, showing a clear but less intense effect on neural stem cells due to a lower concentration of toxic breakdown products, as well as CSF’s growth factors, nutrients and anti-inflammatory proteins.
“This is one of the most complex in vitro models I have constructed and seen,” Herland says. “That we could recapitulate all these interactions is amazing. That we can then see relevant responses to both simulated conditions and patients’ samples is really important, as there is currently no established treatment for these patients.
Going forward, the team aims to use the platform for studying different levels of injury and scaling up the model. “We hope to screen more treatments that could be even more effective than the one we studied.”
David Callahan

