Heart ‘blueprint’ reveals origins of defects and insights into fetal development

New research has produced a “blueprint” revealing how the human heart is built during prenatal development. It offers insights that could lead to improved prenatal care and new treatments for heart defects, such as holes between heart chambers or deformities of the heart valves.
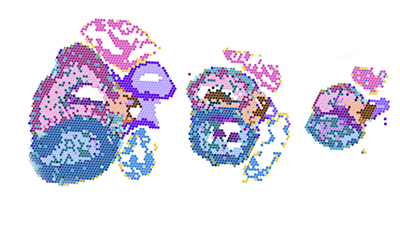
Publishing in Nature Genetics, a research team led by the department of Gene Technology at KTH Royal Institute of Technology published a detailed map of the developing human heart, showing how different groups of cells are arranged and how they interact in fetal heart development.
“Congenital heart diseases, and several acquired ones, originate during early development, which highlights the importance of this period in defining a healthy heart,” says Enikő Lázár, co-lead author of the study.
“This map provides a kind of blueprint, showing how key parts of the heart—like the pacemaker system, heart valves, and the wall between the upper chambers—form and function,” Lázár says.
Among the findings was the discovery of a previously unknown group of cells that produce adrenaline. Lázár says these cells are likely unique to humans and may help the heart respond to low oxygen levels during development or birth. This may explain how the fetal heart adapts to stress and indicates a possible origin of rare heart tumors called pheochromocytomas, the researchers reported.
KTH Professor Joakim Lundeberg, who led the research team, says the work was carried out with cutting-edge technology invented at KTH that allows researchers to study the activity of all genes in human tissue.
This method of spatial transcriptomics enables visualization and analysis of the full range of messenger RNA, or mRNA, molecules expressed by an organism, says Raphaël Mauron, co-lead author of the study. “With careful bioinformatic analysis, we can learn details of heart development that were not possible even a few years ago,” Raphaël Mauron says.
Drawing on the Developmental Tissue Bank of Karolinska Institutet, the map identifies more than 70 distinct cell types, revealing a surprising variety among certain support cells and tracking how nerve connections begin to form.
Other key insights reported:
-
The cells forming heart valves and the atrial septum are more diverse, which offers clues to how the heart’s internal structures form and why certain congenital heart defects—like valve malformations or holes between chambers—occur.
-
The fetal heart has an unexpected variety of supporting cells, or mesenchymal cells, which provide a kind of scaffolding to help shape the heart’s structure and may even be involved in diseases like valve defects or arrhythmias.
-
A precise map of the wiring of cells that form the heart’s natural pacemaker and conduction system, including the sinoatrial node, which sets the heartbeat, and Purkinje fibers, which spread the signal.
-
The map also traces how nerve cells and their support cells grow into the heart and connect with pacemaker cells, revealing that different types of their signals—like noradrenaline and acetylcholine—start influencing the heart early on.
The findings are available through an interactive online tool, offering a valuable resource for understanding heart development and its links to genetic heart conditions.
The study was conducted by researchers from KTH Royal Institute of Technology, Karolinska Institutet, and Stockholm University through the joint research center Science for Life Laboratory (SciLifeLab), as part of the Human Developmental Cell Atlas initiative funded by the Erling Persson Foundation and Knut and Alice Wallenberg Foundation.
David Callahan



